Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity
- PMID: 27461084
- PMCID: PMC5399229
- DOI: 10.1038/npp.2016.134
Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity
Abstract
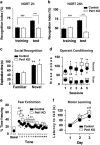
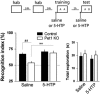
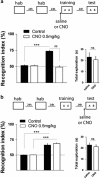
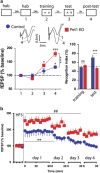
Serotonin (5-HT) deficiency occurs in a number of brain disorders that affect cognitive function. However, a direct causal relationship between 5-HT hypo-transmission and memory and underlying mechanisms has not been established. We used mice with a constitutive depletion of 5-HT brain levels (Pet1KO mice) to analyze the contribution of 5-HT to different forms of learning and memory. Pet1KO mice exhibited a striking deficit in novel object recognition memory, a hippocampal-dependent task. No alterations were found in tasks for social recognition, procedural learning, or fear memory. Viral delivery of designer receptors exclusively activated by designer drugs was used to selectively silence the activity of 5-HT neurons in the raphe. Inhibition of 5-HT neurons in the median raphe, but not the dorsal raphe, was sufficient to impair object recognition in adult mice. In vivo electrophysiology in behaving mice showed that long-term potentiation in the hippocampus of 5-HT-deficient mice was altered, and administration of the 5-HT1A agonist 8-OHDPAT rescued the memory deficits. Our data suggest that hyposerotonergia selectively affects declarative hippocampal-dependent memory. Serotonergic projections from the median raphe are necessary to regulate object memory and hippocampal synaptic plasticity processes, through an inhibitory control mediated by 5-HT1A receptors.
Figures

References
-
- Adell A, Carceller A, Artigas F (1993). In vivo brain dialysis study of the somatodendritic release of serotonin in the Raphe nuclei of the rat: effects of 8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem 60: 1673–1681. - PubMed
-
- Babar E, Melik E, Ozgünen T, Polat S (2002). Effects of excitotoxic median raphe lesion on working memory deficits produced by the dorsal hippocampal muscarinic receptor blockade in the inhibitory avoidance in rats. Brain Res Bull 57: 683–688. - PubMed
-
- Balleine BW, Liljeholm M, Ostlund SB (2009). The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 199: 43–52. - PubMed
-
- Barnes NM, Sharp T (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. - PubMed
-
- Bielsky IF, Young LJ (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides 25: 1565–1574. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

