Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study
- PMID: 27461166
- PMCID: PMC4962457
- DOI: 10.1186/s12883-016-0635-y
Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study
Abstract
Background: Daclizumab is a humanized monoclonal antibody against CD25 that modulates interleukin 2 signaling. The SELECT TRILOGY of clinical studies (SELECT/SELECTION/SELECTED) evaluated the safety and efficacy of daclizumab in patients with relapsing-remitting multiple sclerosis (RRMS). We report the long-term safety and efficacy of daclizumab 150 mg subcutaneous every 4 weeks in patients with RRMS in the SELECTED open-label extension study.
Methods: An interim intent-to-treat analysis of all enrolled patients was performed in January 2014 for this ongoing study.
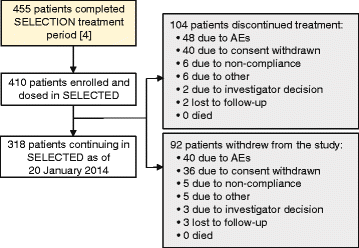
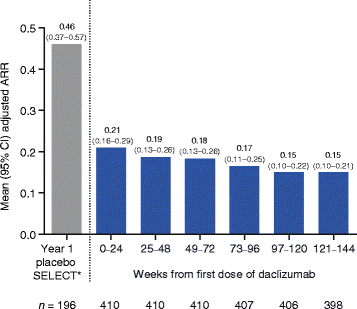
Results: The SELECTED study enrolled 90% of patients who completed SELECTION. In the safety and efficacy analysis (N = 410), median treatment time in SELECTED was 25 months (range, <1-45). Adverse events (AEs) were reported in 76% of patients, serious AEs (SAEs) excluding MS relapse in 16%, and treatment discontinuation due to AEs including multiple sclerosis (MS) relapse in 12%. AEs were primarily of mild to moderate severity, and common AEs (≥10%), excluding MS relapse, were nasopharyngitis (12%) and upper respiratory tract infection (12%). Most commonly reported SAEs (in ≥3 patients), excluding MS relapses, were increased serum hepatic enzymes, pneumonia, ulcerative colitis, and urinary tract infection (<1% each). Incidences of AE groups of interest include cutaneous events (28%), cutaneous SAEs (2%), gastrointestinal SAEs (2%), hepatic SAEs, (1%) and malignancies (1%). The incidence of AEs, SAEs, and treatment-related study discontinuations did not increase over time and no deaths were reported. The adjusted annualized relapse rate (95% confidence interval (CI)) analyzed at 6-month intervals was 0.15 (0.10-0.22) for weeks 97-120 and 0.15 (0.10-0.21) for weeks 121-144. In year 3, the adjusted mean (95% CI) number of new/newly enlarging T2 hyperintense lesions was 1.26 (0.93-1.72) and the mean (median) annualized change in brain volume was -0.32% (-0.34%).
Conclusions: The AE incidence did not increase with extension of therapy into year 3 in SELECTED; the safety profile was similar to that previously observed. The clinical efficacy of daclizumab was sustained over the 3 years comprising the SELECT TRILOGY, although potential selection bias cannot be excluded.
Trial registration: Clinicaltrials.gov NCT01051349; first registered January 15, 2010.
Keywords: Daclizumab; Efficacy; Relapsing-remitting multiple sclerosis; Safety.
Figures



References
-
- World Health Organization. Global Tuberculosis Report 2013 [Ukraine country profile]. http://www.who.int/tb/country/data/profiles/en/. Accessed 12 October 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

