Ribonuclease H1-dependent hepatotoxicity caused by locked nucleic acid-modified gapmer antisense oligonucleotides
- PMID: 27461380
- PMCID: PMC4961955
- DOI: 10.1038/srep30377
Ribonuclease H1-dependent hepatotoxicity caused by locked nucleic acid-modified gapmer antisense oligonucleotides
Abstract
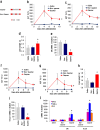
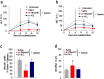
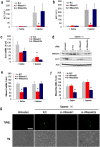
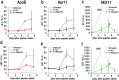
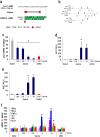
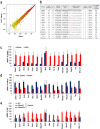
Gapmer antisense oligonucleotides cleave target RNA effectively in vivo, and is considered as promising therapeutics. Especially, gapmers modified with locked nucleic acid (LNA) shows potent knockdown activity; however, they also cause hepatotoxic side effects. For developing safe and effective gapmer drugs, a deeper understanding of the mechanisms of hepatotoxicity is required. Here, we investigated the cause of hepatotoxicity derived from LNA-modified gapmers. Chemical modification of gapmer's gap region completely suppressed both knockdown activity and hepatotoxicity, indicating that the root cause of hepatotoxicity is related to intracellular gapmer activity. Gene silencing of hepatic ribonuclease H1 (RNaseH1), which catalyses gapmer-mediated RNA knockdown, strongly supressed hepatotoxic effects. Small interfering RNA (siRNA)-mediated knockdown of a target mRNA did not result in any hepatotoxic effects, while the gapmer targeting the same position on mRNA as does the siRNA showed acute toxicity. Microarray analysis revealed that several pre-mRNAs containing a sequence similar to the gapmer target were also knocked down. These results suggest that hepatotoxicity of LNA gapmer is caused by RNAseH1 activity, presumably because of off-target cleavage of RNAs inside nuclei.
Figures
References
-
- Crook S. T. Antisense Drug Technology: Principles, Strategies, and Applications, 2nd edition. (CRC Press, 2008).
-
- Bennett F. C. & Swayze E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010). - PubMed
-
- Geary R. S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 5, 381–391 (2009). - PubMed
-
- Obika S., Morio K., Nanbu D. & Imanishi T. Synthesis and conformation of 3′-O,4′-C-methyleneribonucleosides, novel bicyclic nucleoside analogues for 2′, 5′-linked oligonucleotide modification. Chem. Commun. 17, 1643–1644 (1997).
-
- Koshkin A. A. et al. LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 54, 3607–3630 (1998).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

