Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins
- PMID: 27461578
- PMCID: PMC5872827
- DOI: 10.1021/acs.chemrev.6b00334
Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins
Abstract
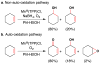
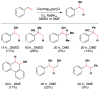
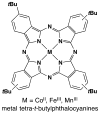
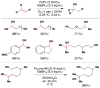
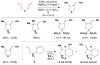
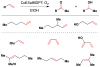
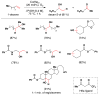
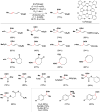
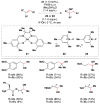
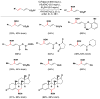
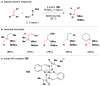
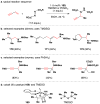
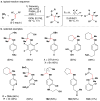
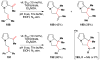
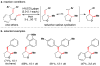
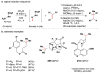
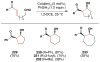
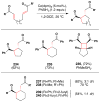
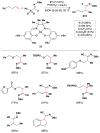
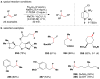
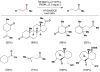
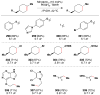
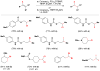
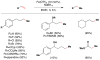
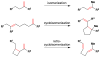
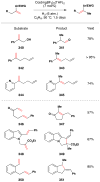
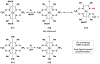
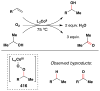
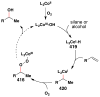
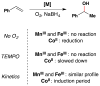
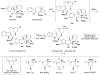
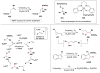
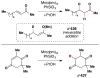
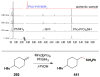
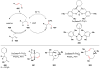
Cofactor-mimetic aerobic oxidation has conceptually merged with catalysis of syngas reactions to form a wide range of Markovnikov-selective olefin radical hydrofunctionalizations. We cover the development of the field and review contributions to reaction invention, mechanism, and application to complex molecule synthesis. We also provide a mechanistic framework for understanding this compendium of radical reactions.
Figures






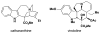


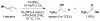

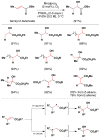
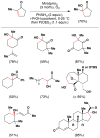


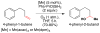


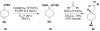

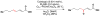

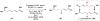
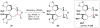
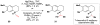

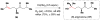

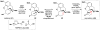
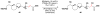
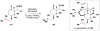

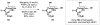
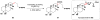

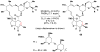
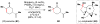

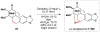

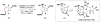
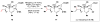
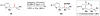
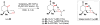


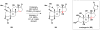
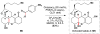

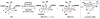




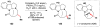
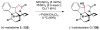
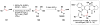
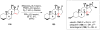

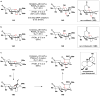











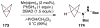
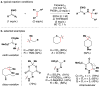
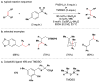
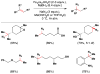
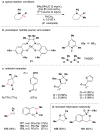


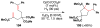



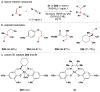
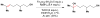
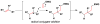
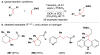
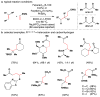
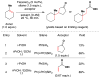
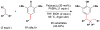
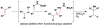



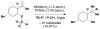



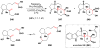
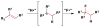
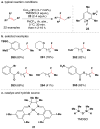

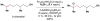
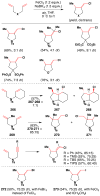

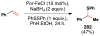




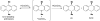


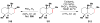

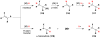
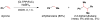
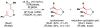

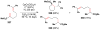
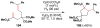
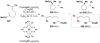
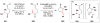
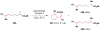











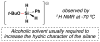





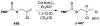
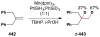


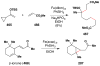
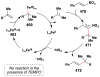
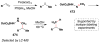




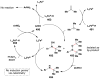



References
-
- Hegedus LS. Transition Metals in the Synthesis of Complex Organic Molecules. University Science Books; Sausalito, CA: 1999.
-
- Mathey F. Transition Metal Organometallic Chemistry. Springer; New York, NY: 2013.
-
- Collman JP, Hegedus LS, Norton JR, Finke RG. Principles and Applications of Organotransition Metal Chemistry. University Science Books; Mill Valley, CA: 1987.
-
- Hartung J, Norton J. In: Catalysis Without Precious Metals. Bullock RM, editor. Wiley; Weinheim: 2010.
-
- Hoffmann RW. Markovnikov Free Radical Addition Reactions, a Sleeping Beauty Kissed to Life. Chem Soc Rev. 2016;45:577–583. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

