Smoking, pregnancy and the subgingival microbiome
- PMID: 27461975
- PMCID: PMC4961950
- DOI: 10.1038/srep30388
Smoking, pregnancy and the subgingival microbiome
Abstract
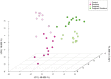
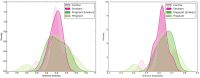
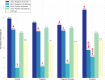
The periodontal microbiome is known to be altered during pregnancy as well as by smoking. However, despite the fact that 2.1 million women in the United States smoke during their pregnancy, the potentially synergistic effects of smoking and pregnancy on the subgingival microbiome have never been studied. Subgingival plaque was collected from 44 systemically and periodontally healthy non-pregnant nonsmokers (control), non-pregnant smokers, pregnant nonsmokers and pregnant smokers and sequenced using 16S-pyrotag sequencing. 331601 classifiable sequences were compared against HOMD. Community ordination methods and co-occurrence networks were used along with non-parametric tests to identify differences between groups. Linear Discriminant Analysis revealed significant clustering based on pregnancy and smoking status. Alpha diversity was similar between groups, however, pregnant women (smokers and nonsmokers) demonstrated higher levels of gram-positive and gram-negative facultatives, and lower levels of gram-negative anaerobes when compared to smokers. Each environmental perturbation induced distinctive co-occurrence patterns between species, with unique network anchors in each group. Our study thus suggests that the impact of each environmental perturbation on the periodontal microbiome is unique, and that when they are superimposed, the sum is greater than its parts. The persistence of these effects following cessation of the environmental disruption warrants further investigation.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

