Limited effects of preterm birth and the first enteral nutrition on cerebellum morphology and gene expression in piglets
- PMID: 27462071
- PMCID: PMC4962075
- DOI: 10.14814/phy2.12871
Limited effects of preterm birth and the first enteral nutrition on cerebellum morphology and gene expression in piglets
Erratum in
-
Erratum.Physiol Rep. 2016 Aug;4(16):e12954. doi: 10.14814/phy2.12954. Physiol Rep. 2016. PMID: 27561912 Free PMC article. No abstract available.
Abstract
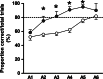
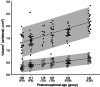
Preterm pigs show many signs of immaturity that are characteristic of preterm infants. In preterm infants, the cerebellum grows particularly rapid and hypoplasia and cellular lesions are associated with motor dysfunction and cognitive deficits. We hypothesized that functional brain delays observed in preterm pigs would be paralleled by both structural and molecular differences in the cerebellum relative to term born piglets. Cerebella were collected from term (n = 56) and preterm (90% gestation, n = 112) pigs at 0, 5, and 26 days after birth for stereological volume estimations, large-scale qPCR gene expression analyses (selected neurodevelopmental genes) and western blot protein expression analysis (Sonic Hedgehog pathway). Memory and learning was tested using a T-maze, documenting that preterm pigs showed delayed learning. Preterm pigs also showed reduced volume of both white and gray matter at all three ages but the proportion of white matter increased postnatally, relative to term pigs. Early initiation of enteral nutrition had limited structural or molecular effects. The Sonic Hedgehog pathway was unaffected by preterm birth. Few differences in expression of the selected genes were found, except consistently higher mRNA levels of Midkine, p75, and Neurotrophic factor 3 in the preterm cerebellum postnatally, probably reflecting an adaptive response to preterm birth. Pig cerebellar development appears more affected by postconceptional age than by environmental factors at birth or postnatally. Compensatory mechanisms following preterm birth may include faster white matter growth and increased expression of selected genes for neurotrophic factors and regulation of angiogenesis. While the pig cerebellum is immature in 90% gestation preterm pigs, it appears relatively mature and resilient toward environmental factors.
Keywords: Enteral and parenteral nutrition; neonatal brain development; postconceptional age; prematurity.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures


References
-
- Allais, A. , Burel D., Isaac E. R., Gray S. L., Basille M., Ravni A., et al. 2007. Altered cerebellar development in mice lacking pituitary adenylate cyclase‐activating polypeptide. Eur. J. Neurosci. 25:2604–2618. - PubMed
-
- Andersen, C. L. , Jensen J. L., and Orntoft T. F.. 2004. Normalization of real‐time quantitative reverse transcription‐PCR data: a model‐based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64:5245–5250. - PubMed
-
- Andersen, A. D. , Sangild P. T., Munch S. L., van der Beek E. M., Renes I. B., van Ginneken C., et al., 2016. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am. J. Physiol. Regul. Integr. Comp. Physiol., 310, R481–R492. ajpregu.00349.2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

