Cell Damage in Light Chain Amyloidosis: FIBRIL INTERNALIZATION, TOXICITY AND CELL-MEDIATED SEEDING
- PMID: 27462073
- PMCID: PMC5025671
- DOI: 10.1074/jbc.M116.736736
Cell Damage in Light Chain Amyloidosis: FIBRIL INTERNALIZATION, TOXICITY AND CELL-MEDIATED SEEDING
Abstract
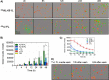
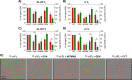
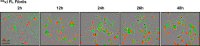
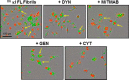
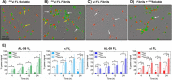
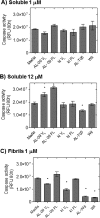
Light chain (AL) amyloidosis is an incurable human disease characterized by the misfolding, aggregation, and systemic deposition of amyloid composed of immunoglobulin light chains (LC). This work describes our studies on potential mechanisms of AL cytotoxicity. We have studied the internalization of AL soluble proteins and amyloid fibrils into human AC16 cardiomyocytes by using real time live cell image analysis. Our results show how external amyloid aggregates rapidly surround the cells and act as a recruitment point for soluble protein, triggering the amyloid fibril elongation. Soluble protein and external aggregates are internalized into AC16 cells via macropinocytosis. AL amyloid fibrils are shown to be highly cytotoxic at low concentrations. Additionally, caspase assays revealed soluble protein induces apoptosis, demonstrating different cytotoxic mechanisms between soluble protein and amyloid aggregates. This study emphasizes the complex immunoglobulin light chain-cell interactions that result in fibril internalization, protein recruitment, and cytotoxicity that may occur in AL amyloidosis.
Keywords: amyloid; apoptosis; cardiomyocytes; cell internalization; endocytosis; fibril fragmentation; in vivo imaging; light chain amyloidosis; protein aggregation; toxicity.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Falk R. H. (2005) Diagnosis and management of the cardiac amyloidoses. Circulation 112, 2047–2060 - PubMed
-
- Kumar S. K., Gertz M. A., Lacy M. Q., Dingli D., Hayman S. R., Buadi F. K., Short-Detweiler K., Zeldenrust S. R., Leung N., Greipp P. R., Lust J. A., Russell S. J., Kyle R. A., Rajkumar S. V., and Dispenzieri A. (2011) Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin. Proc. 86, 12–18 - PMC - PubMed
-
- Glenner G. G., Cuatrecasas P., Isersky C., Bladen H. A., and Eanes E. D. (1969) Physical and chemical properties of amyloid fibers: II. Isolation of a unique protein constituting the major component from human splenic amyloid fibril concentrates. J. Histochem. Cytochem. 17, 769–780 - PubMed
-
- Olsen K. E., Sletten K., and Westermark P. (1998) Fragments of the constant region of immunoglobulin light chains are constituents of AL-amyloid proteins. Biochem. Biophys. Res. Commun. 251, 642–647 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

