Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth
- PMID: 27462085
- PMCID: PMC5074613
- DOI: 10.1104/pp.16.00530
Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth
Abstract
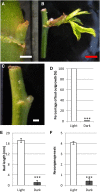
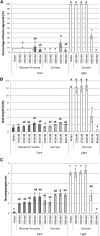
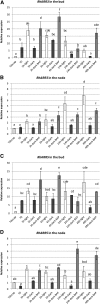
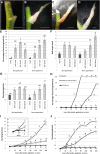
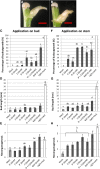
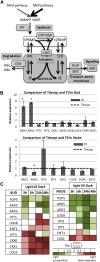
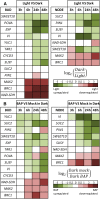
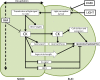
Bud outgrowth is controlled by environmental and endogenous factors. Through the use of the photosynthesis inhibitor norflurazon and of masking experiments, evidence is given here that light acts mainly as a morphogenic signal in the triggering of bud outgrowth and that initial steps in the light signaling pathway involve cytokinins (CKs). Indeed, in rose (Rosa hybrida), inhibition of bud outgrowth by darkness is suppressed solely by the application of CKs. In contrast, application of sugars has a limited effect. Exposure of plants to white light (WL) induces a rapid (after 3-6 h of WL exposure) up-regulation of CK synthesis (RhIPT3 and RhIPT5), of CK activation (RhLOG8), and of CK putative transporter RhPUP5 genes and to the repression of the CK degradation RhCKX1 gene in the node. This leads to the accumulation of CKs in the node within 6 h and in the bud at 24 h and to the triggering of bud outgrowth. Molecular analysis of genes involved in major mechanisms of bud outgrowth (strigolactone signaling [RwMAX2], metabolism and transport of auxin [RhPIN1, RhYUC1, and RhTAR1], regulation of sugar sink strength [RhVI, RhSUSY, RhSUC2, and RhSWEET10], and cell division and expansion [RhEXP and RhPCNA]) reveal that, when supplied in darkness, CKs up-regulate their expression as rapidly and as intensely as WL Additionally, up-regulation of CKs by WL promotes xylem flux toward the bud, as evidenced by Methylene Blue accumulation in the bud after CK treatment in the dark. Altogether, these results suggest that CKs are initial components of the light signaling pathway that controls the initiation of bud outgrowth.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Alberts AW. (1988) Discovery, biochemistry and biology of lovastatin. Am J Cardiol 62: 10J–15J - PubMed
-
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65: 571–577 - PubMed
-
- Bangerth F. (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194: 439–442
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

