Peripheral tolerance can be modified by altering KLF2-regulated Treg migration
- PMID: 27462110
- PMCID: PMC4987800
- DOI: 10.1073/pnas.1605849113
Peripheral tolerance can be modified by altering KLF2-regulated Treg migration
Abstract
Tregs are essential for maintaining peripheral tolerance, and thus targeting these cells may aid in the treatment of autoimmunity and cancer by enhancing or reducing suppressive functions, respectively. Before these cells can be harnessed for therapeutic purposes, it is necessary to understand how they maintain tolerance under physiologically relevant conditions. We now report that transcription factor Kruppel-like factor 2 (KLF2) controls naive Treg migration patterns via regulation of homeostatic and inflammatory homing receptors, and that in its absence KLF2-deficient Tregs are unable to migrate efficiently to secondary lymphoid organs (SLOs). Diminished Treg trafficking to SLOs is sufficient to initiate autoimmunity, indicating that SLOs are a primary site for maintaining peripheral tolerance under homeostatic conditions. Disease severity correlates with impaired Treg recruitment to SLOs and, conversely, promotion of Tregs into these tissues can ameliorate autoimmunity. Moreover, stabilizing KLF2 expression within the Treg compartment enhances peripheral tolerance by diverting these suppressive cells from tertiary tissues into SLOs. Taken together, these results demonstrate that peripheral tolerance is enhanced or diminished through modulation of Treg trafficking to SLOs, a process that can be controlled by adjusting KLF2 protein levels.
Keywords: KLF2; Treg migration; autoimmunity; peripheral tolerance; regulatory T cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

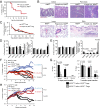

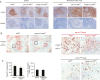

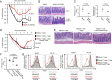
References
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. - PubMed
-
- Bates GJ, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. - PubMed
-
- Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. - PubMed
-
- Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

