ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2
- PMID: 27462118
- PMCID: PMC4995121
- DOI: 10.1161/STROKEAHA.116.012825
ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2
Abstract
Background and purpose: The ALIAS (Albumin in Acute Ischemic Stroke) part 1 and 2 trials evaluated whether 25% human serum albumin improves clinical outcomes after acute ischemic stroke above and beyond standard of care using similar protocols. The part 1 trial ended prematurely because of safety concerns, and the part 2 trial terminated early because of futility of finding a statistically significant effect of albumin over saline (control) administration. We combine the subject-level data of the part 1 and 2 trials to reevaluate the efficacy and safety outcomes with the larger sample size.
Methods: The combined data analyses closely follow those conducted in the part 2 trial. The primary outcome is the composite of the modified Rankin Scale and the National Institutes of Health Stroke Scale defined as a composite of modified Rankin Scale score 0 to 1 and National Institutes of Health Stroke Scale score 0 to 1 at 90 days from randomization. The unadjusted analyses use a simple Chi-square test, and those adjusting for baseline covariates use a generalized linear model with log link (to obtain relative risks).
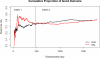
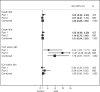
Results: The participant characteristics at baseline were generally similar between the treatment groups and between the trials; however, thrombolysis use was greater in part 2 (84% versus 75%), and the upper age limit imposed in part 2 resulted in a younger sample (mean age in part 1 was 69 versus 64 in part 2). In the combined sample, the proportions of good outcome in the 2 treatment groups were identical (41%). Similar results were observed in all secondary efficacy outcomes. Pulmonary edema was a consistent safety concern, with a 6-fold increase in the albumin arm (13%) compared with saline (2%; relative risk =7.76, 95% confidence interval 3.87-15.57).
Conclusions: Treatment with intravenous albumin 25% at 2 g/kg was not associated with improved outcome at 90 days and was associated with increased rates of intracerebral hemorrhage and pulmonary edema.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT00235495.
Keywords: acute stroke; albumin; randomized trial; risk; serum albumin.
© 2016 American Heart Association, Inc.
Figures
References
-
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: Marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. - PubMed
-
- Ginsberg M, Belayev L, Bazan N, Marcheselli V, Hill M, Palesch Y, et al. Albumin-based neurotherapeutics for acute ischemic stroke: From bench to bedside. In: J K, S K, editors. Pharmacology of cerebral ischemia. Medpharm Scientific Publishers; Stuttgart: 2004. pp. 421–433.
-
- Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. - PubMed
-
- Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–6. - PubMed
-
- Hill MD, Moy CS, Palesch YY, Martin R, Dillon CR, Waldman BD, et al. for the ALIAS Investigators The albumin in acute stroke trial (ALIAS); design and methodology. Int J Stroke. 2007;2:214–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical