Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin
- PMID: 27462268
- PMCID: PMC4940422
- DOI: 10.3389/fphar.2016.00195
Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin
Abstract
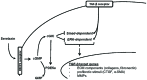
Introduction: Kidney fibrosis has shown to be ameliorated through the involvement of cyclic guanosine monophosphate (cGMP) and its dependent protein kinase I (cGKI). Serelaxin, the recombinant form of human relaxin-II, increases cGMP levels and has shown beneficial effects on kidney function in acute heart failure patients. Antifibrotic properties of serelaxin are supposed to be mediated via relaxin family peptide receptor 1 and subsequently enhanced nitric oxide/cGMP to inhibit transforming growth factor-β (TGF-β) signaling. This study examines the involvement of cGKI in the antifibrotic signaling of serelaxin.
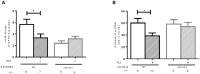
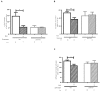
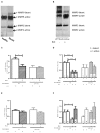
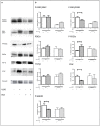
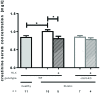
Methods and results: Kidney fibrosis was induced by unilateral ureteral obstruction in wildtype (WT) and cGKI knock-out (KO) mice. After 7 days, renal antifibrotic effects of serelaxin were assessed. Serelaxin treatment for 7 days significantly increased cGMP in the kidney of WT and cGKI-KO. In WT, renal fibrosis was reduced through decreased accumulation of collagen1A1, total collagen, and fibronectin. The profibrotic connective tissue growth factor as well as myofibroblast differentiation were reduced and matrix metalloproteinases-2 and -9 were positively modulated after treatment. Moreover, Smad2 as well as extracellular signal-regulated kinase 1 (ERK1) phosphorylation were decreased, whereas phosphodiesterase (PDE) 5a phosphorylation was increased. However, these effects were not observed in cGKI-KO.
Conclusion: Antifibrotic renal effects of serelaxin are mediated via cGMP/cGKI to inhibit Smad2- and ERK1-dependent TGF-β signaling and increased PDE5a phosphorylation.
Keywords: Relaxin; cGMP-dependent protein kinase; interstitial fibrosis; kidney; nitric oxide; serelaxin; signaling.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

