Activation of Sterol Regulatory Element Binding Factors by Fenofibrate and Gemfibrozil Stimulates Myelination in Zebrafish
- PMID: 27462272
- PMCID: PMC4939524
- DOI: 10.3389/fphar.2016.00206
Activation of Sterol Regulatory Element Binding Factors by Fenofibrate and Gemfibrozil Stimulates Myelination in Zebrafish
Abstract
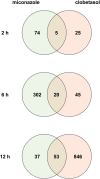
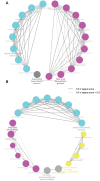
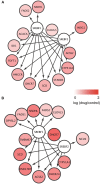
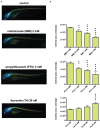
Oligodendrocytes are major myelin-producing cells and play essential roles in the function of a healthy nervous system. However, they are also one of the most vulnerable neural cell types in the central nervous system (CNS), and myelin abnormalities in the CNS are found in a wide variety of neurological disorders, including multiple sclerosis, adrenoleukodystrophy, and schizophrenia. There is an urgent need to identify small molecular weight compounds that can stimulate myelination. In this study, we performed comparative transcriptome analysis to identify pharmacodynamic effects common to miconazole and clobetasol, which have been shown to stimulate myelination by mouse oligodendrocyte progenitor cells (OPCs). Of the genes differentially expressed in both miconazole- and clobetasol-treated mouse OPCs compared with untreated cells, we identified differentially expressed genes (DEGs) common to both drug treatments. Gene ontology analysis revealed that these DEGs are significantly associated with the sterol biosynthetic pathway, and further bioinformatics analysis suggested that sterol regulatory element binding factors (SREBFs) might be key upstream regulators of the DEGs. In silico screening of a public database for chemicals associated with SREBF activation identified fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) agonist, as a drug that increases the expression of known SREBF targets, raising the possibility that fenofibrate may also stimulate myelination. To test this, we performed in vivo imaging of zebrafish expressing a fluorescent reporter protein under the control of the myelin basic protein (mbp) promoter. Treatment of zebrafish with fenofibrate significantly increased expression of the fluorescent reporter compared with untreated zebrafish. This increase was attenuated by co-treatment with fatostatin, a specific inhibitor of SREBFs, confirming that the fenofibrate effect was mediated via SREBFs. Furthermore, incubation of zebrafish with another PPARα agonist, gemfibrozil, also increased expression of the mbp promoter-driven fluorescent reporter in an SREBF-dependent manner. These results suggest that activation of SREBFs by small molecular weight compounds may be a feasible therapeutic approach to stimulate myelination.
Keywords: SREBFs; comparative transcriptomics; fenofibrate; gemfibrozil; myelination; oligodendrocytes; systems pharmacology; zebrafish.
Figures
Similar articles
-
Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish.Exp Neurol. 2014 Jan;251:72-83. doi: 10.1016/j.expneurol.2013.11.012. Epub 2013 Nov 18. Exp Neurol. 2014. PMID: 24262204
-
Evaluation of the therapeutic potential of PPARalpha agonists for X-linked adrenoleukodystrophy.Mol Genet Metab. 2003 Dec;80(4):398-407. doi: 10.1016/j.ymgme.2003.09.002. Mol Genet Metab. 2003. PMID: 14654352
-
Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo.Nature. 2015 Jun 11;522(7555):216-20. doi: 10.1038/nature14335. Epub 2015 Apr 20. Nature. 2015. PMID: 25896324 Free PMC article.
-
Insights into mechanisms of central nervous system myelination using zebrafish.Glia. 2016 Mar;64(3):333-49. doi: 10.1002/glia.22897. Epub 2015 Aug 6. Glia. 2016. PMID: 26250418 Review.
-
Imaging Myelination In Vivo Using Transparent Animal Models.Brain Plast. 2016 Dec 21;2(1):3-29. doi: 10.3233/BPL-160029. Brain Plast. 2016. PMID: 29765846 Free PMC article. Review.
Cited by
-
Targeting Smoothened as a New Frontier in the Functional Recovery of Central Nervous System Demyelinating Pathologies.Int J Mol Sci. 2018 Nov 20;19(11):3677. doi: 10.3390/ijms19113677. Int J Mol Sci. 2018. PMID: 30463396 Free PMC article. Review.
-
The Current Challenges for Drug Discovery in CNS Remyelination.Int J Mol Sci. 2021 Mar 12;22(6):2891. doi: 10.3390/ijms22062891. Int J Mol Sci. 2021. PMID: 33809224 Free PMC article. Review.
-
Generation of a Triple-Transgenic Zebrafish Line for Assessment of Developmental Neurotoxicity during Neuronal Differentiation.Pharmaceuticals (Basel). 2019 Sep 24;12(4):145. doi: 10.3390/ph12040145. Pharmaceuticals (Basel). 2019. PMID: 31554324 Free PMC article.
-
Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review.Int J Mol Sci. 2020 Oct 30;21(21):8116. doi: 10.3390/ijms21218116. Int J Mol Sci. 2020. PMID: 33143194 Free PMC article. Review.
-
Role of fenofibrate in multiple sclerosis.Eur J Med Res. 2024 Feb 9;29(1):113. doi: 10.1186/s40001-024-01700-2. Eur J Med Res. 2024. PMID: 38336772 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

