Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question
- PMID: 27462361
- PMCID: PMC4960900
- DOI: 10.1186/s12981-016-0111-1
Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question
Abstract
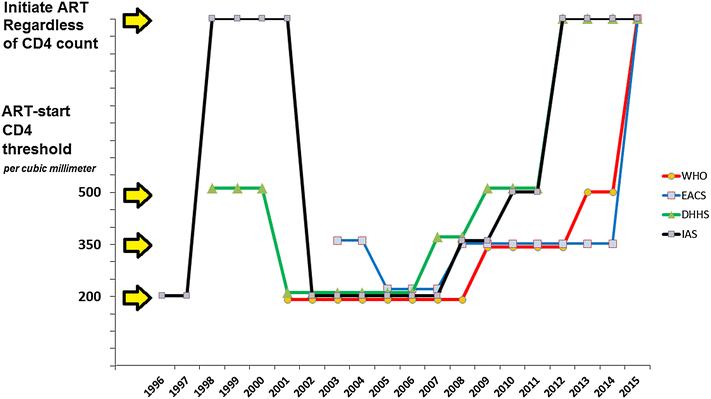
After a period where it was recommended to start antiretroviral therapy (ART) early, the CD4 threshold for treating asymptomatic adults dropped to 200/mm(3) at the beginning of the 2000s. This was mostly due to a great prudence with regards to drug toxicity. The ART-start CD4 threshold in most international guidelines was then raised to 350/mm(3) in 2006-2009 and to 500/mm(3) in 2009-2013. Between 2012 and 2015, international guidelines went the last step further and recommended treating all HIV-infected adults regardless of their CD4 count. This ultimate step was justified by the results of three randomized controlled trials, HPTN 052, Temprano ANRS 12136 and START. These three trials assessed the benefits and risks of starting ART immediately upon inclusion ("early ART") versus deferring ART until the current starting criteria were met ("deferred ART"). Taken together, they recruited 8427 HIV-infected adults in 37 countries. The primary outcome was severe morbidity, a composite outcome that included all-cause deaths, AIDS diseases, and non-AIDS cancers in the three trials. The trial results were mutually consistent and reinforcing. The overall risk of severe morbidity was significantly 44-57 % lower in patients randomized to early ART as compared to deferred ART. Early ART also decreased the risk of AIDS, tuberculosis, invasive bacterial diseases and Kaposi's sarcoma considered separately. The incidence of severe morbidity was 3.2 and 3.5 times as high in HPTN052 and Temprano as in START, respectively. This difference is mostly due to the geographical context of morbidity. The evidence is now strong that initiating ART at high CD4 counts entails individual benefits worldwide, and that this is all the more true in low resource contexts where tuberculosis and other bacterial diseases are highly prevalent. These benefits in addition to population benefits consisting of preventing HIV transmission demonstrated in HPTN052, justify the recommendation that HIV-infected persons should initiate ART regardless of CD4 count. This recommendation faces many challenges, including the fact that switching from "treat at 500 CD4/mm(3)" to "treat everyone" not only requires more tests and more drugs, but also more people to support patients and help them remain in care.
Keywords: Early antiretroviral treatment; Randomized controlled trial.
Figures

References
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. - DOI - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

