Pyridoxamine is a substrate of the energy-coupling factor transporter HmpT
- PMID: 27462413
- PMCID: PMC4860826
- DOI: 10.1038/celldisc.2015.14
Pyridoxamine is a substrate of the energy-coupling factor transporter HmpT
Abstract
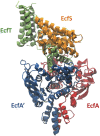
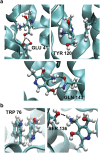
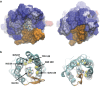
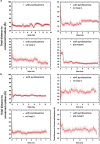
Energy-coupling factor (ECF) transporters belong to a novel family of proteins that forms a subset within the ATP-binding cassette (ABC) transporter family. These proteins are responsible for the uptake of micronutrients in bacteria. ECF transporters are composed of four proteins: the A- and A'-components, the T-component and the S-component. One of the ECF transporters, named HmpT, was crystallized in the apo form with all four components. It is currently unknown whether HmpT serves as a transporter for hydroxymethyl pyrimidine or the different forms of vitamin B6 (pyridoxine, pyridoxal or pyridoxamine). Using a combination of molecular dynamics (MD) simulations and mass spectrometry, we have identified pyridoxamine to be the preferred substrate of HmpT. Mass spectra show that the mass of the substrate from the HmpT-substrate complex matches that of pyridoxamine. MD simulations likewise indicate that pyridoxamine interacts most strongly with most of the conserved residues of the S-component (Glu 41, His 84 and Gln 43) compared with the other vitamin B6 forms. Furthermore, the simulations have implied that loops 1 and 5 of the S-component can participate in the gating action for HmpT.
Keywords: ECF transporters; HmpT; S-component; gating mechanism; mass spectrometry; molecular dynamics.
Figures


Similar articles
-
Structure of a pantothenate transporter and implications for ECF module sharing and energy coupling of group II ECF transporters.Proc Natl Acad Sci U S A. 2014 Dec 30;111(52):18560-5. doi: 10.1073/pnas.1412246112. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512487 Free PMC article.
-
Structure and mechanism of a group-I cobalt energy coupling factor transporter.Cell Res. 2017 May;27(5):675-687. doi: 10.1038/cr.2017.38. Epub 2017 Mar 21. Cell Res. 2017. PMID: 28322252 Free PMC article.
-
Energy coupling factor-type ABC transporters for vitamin uptake in prokaryotes.Biochemistry. 2012 Jun 5;51(22):4390-6. doi: 10.1021/bi300504v. Epub 2012 May 21. Biochemistry. 2012. PMID: 22574898 Review.
-
Assembly and mechanism of a group II ECF transporter.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2534-9. doi: 10.1073/pnas.1217361110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359690 Free PMC article.
-
ECF-Type ATP-Binding Cassette Transporters.Annu Rev Biochem. 2019 Jun 20;88:551-576. doi: 10.1146/annurev-biochem-013118-111705. Epub 2019 Nov 28. Annu Rev Biochem. 2019. PMID: 30485755 Review.
Cited by
-
Pyridoxal 5'-phosphate synthesis and salvage in Bacteria and Archaea: predicting pathway variant distributions and holes.Microb Genom. 2023 Feb;9(2):mgen000926. doi: 10.1099/mgen.0.000926. Microb Genom. 2023. PMID: 36729913 Free PMC article.
-
Cytosolic and mitochondrial tRNA synthetase inhibitors increase lifespan in a GCN4/atf-4-dependent manner.iScience. 2022 Oct 21;25(11):105410. doi: 10.1016/j.isci.2022.105410. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388960 Free PMC article.
-
An Aromatic Cap Seals the Substrate Binding Site in an ECF-Type S Subunit for Riboflavin.J Mol Biol. 2016 Jul 31;428(15):3118-30. doi: 10.1016/j.jmb.2016.06.003. Epub 2016 Jun 13. J Mol Biol. 2016. PMID: 27312125 Free PMC article.
-
Knowns and Unknowns of Vitamin B6 Metabolism in Escherichia coli.EcoSal Plus. 2021 Apr;9(2):eESP-0004-2021. doi: 10.1128/ecosalplus.ESP-0004-2021. EcoSal Plus. 2021. PMID: 33787481 Free PMC article. Review.
-
An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis.Elife. 2020 Jun 9;9:e57322. doi: 10.7554/eLife.57322. Elife. 2020. PMID: 32515736 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

