Cell cycle-dependent inhibition of 53BP1 signaling by BRCA1
- PMID: 27462418
- PMCID: PMC4860855
- DOI: 10.1038/celldisc.2015.19
Cell cycle-dependent inhibition of 53BP1 signaling by BRCA1
Abstract
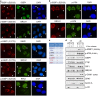
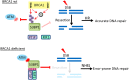
DNA damage response mediator protein 53BP1 is a key regulator of non-homologous end-joining (NHEJ) repair. 53BP1 protects DNA broken ends from resection by recruiting two downstream factors, RIF1 (RAP1-interacting factor 1) and PTIP (Pax transactivation domain-interacting protein), to double-stranded breaks (DSBs) via ATM (ataxia telangiectasia mutated)-mediated 53BP1 phosphorylation, and competes with BRCA1-mediated homologous recombination (HR) repair in G1 phase. In contrast, BRCA1 antagonizes 53BP1-direct NHEJ repair in S/G2 phases. We and others have found that BRCA1 prevents the translocation of RIF1 to DSBs in S/G2 phases; however, the underlying mechanism remains unclear. Here we show that efficient ATM-dependent 53BP1 phosphorylation is restricted to the G1 phase of the cell cycle, as a consequence RIF1 and PTIP accumulation at DSB sites only occur in G1 phase. Mechanistically, both BRCT and RING domains of BRCA1 are required for the inhibition of 53BP1 phosphorylation in S and G2 phases. Thus, our findings reveal how BRCA1 antagonizes 53BP1 signaling to ensure that HR repair is the dominant repair pathway in S/G2 phases.
Keywords: 53BP1; ATM; BRCA1; DNA repair choice; PTIP; RIF1; cell cycle; homologous recombination.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

