Nuclear localization of platelet-activating factor receptor controls retinal neovascularization
- PMID: 27462464
- PMCID: PMC4941644
- DOI: 10.1038/celldisc.2016.17
Nuclear localization of platelet-activating factor receptor controls retinal neovascularization
Erratum in
-
Erratum: Nuclear localization of platelet-activating factor receptor controls retinal neovascularization.Cell Discov. 2016 Sep 20;2:16034. doi: 10.1038/celldisc.2016.34. eCollection 2016. Cell Discov. 2016. PMID: 27672445 Free PMC article.
Abstract
Platelet-activating factor (PAF) is a pleiotropic phospholipid with proinflammatory, procoagulant and angiogenic actions on the vasculature. We and others have reported the presence of PAF receptor (Ptafr) at intracellular sites such as the nucleus. However, mechanisms of localization and physiologic functions of intracellular Ptafr remain poorly understood. We hereby identify the importance of C-terminal motif of the receptor and uncover novel roles of Rab11a GTPase and importin-5 in nuclear translocation of Ptafr in primary human retinal microvascular endothelial cells. Nuclear localization of Ptafr is independent of exogenous PAF stimulation as well as intracellular PAF biosynthesis. Moreover, nuclear Ptafr is responsible for the upregulation of unique set of growth factors, including vascular endothelial growth factor, in vitro and ex vivo. We further corroborate the intracrine PAF signaling, resulting in angiogenesis in vivo, using Ptafr antagonists with distinct plasma membrane permeability. Collectively, our findings show that nuclear Ptafr translocates in an agonist-independent manner, and distinctive functions of Ptafr based on its cellular localization point to another dimension needed for pharmacologic selectivity of drugs.
Keywords: Ptafr; angiogenesis; importin; nuclear GPCR; rab GTPase.
Figures

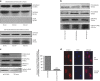

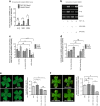

References
-
- Barbaro JF, Zvaifler NJ. Antigen induced histamine release from platelets of rabbits producing homologous PCA antibody. Proc Soc Exp Biol Med 1966; 122: 1245–1247. - PubMed
-
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 1995; 377: 435–438. - PubMed
-
- Harris ES, Rondina MT, Schwertz H, Weyrich AS, Zimmerman GA. Pathogenesis of sepsis and sepsis-induced acute lung injury. Acute Respiratory Distress Syndrome 2010; 2: 369–419.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

