A trans-synaptic nanocolumn aligns neurotransmitter release to receptors
- PMID: 27462810
- PMCID: PMC5002394
- DOI: 10.1038/nature19058
A trans-synaptic nanocolumn aligns neurotransmitter release to receptors
Abstract
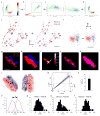
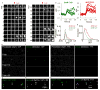
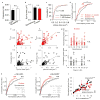
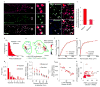
Synaptic transmission is maintained by a delicate, sub-synaptic molecular architecture, and even mild alterations in synapse structure drive functional changes during experience-dependent plasticity and pathological disorders. Key to this architecture is how the distribution of presynaptic vesicle fusion sites corresponds to the position of receptors in the postsynaptic density. However, while it has long been recognized that this spatial relationship modulates synaptic strength, it has not been precisely described, owing in part to the limited resolution of light microscopy. Using localization microscopy, here we show that key proteins mediating vesicle priming and fusion are mutually co-enriched within nanometre-scale subregions of the presynaptic active zone. Through development of a new method to map vesicle fusion positions within single synapses in cultured rat hippocampal neurons, we find that action-potential-evoked fusion is guided by this protein gradient and occurs preferentially in confined areas with higher local density of Rab3-interacting molecule (RIM) within the active zones. These presynaptic RIM nanoclusters closely align with concentrated postsynaptic receptors and scaffolding proteins, suggesting the existence of a trans-synaptic molecular 'nanocolumn'. Thus, we propose that the nanoarchitecture of the active zone directs action-potential-evoked vesicle fusion to occur preferentially at sites directly opposing postsynaptic receptor-scaffold ensembles. Remarkably, NMDA receptor activation triggered distinct phases of plasticity in which postsynaptic reorganization was followed by trans-synaptic nanoscale realignment. This architecture suggests a simple organizational principle of central nervous system synapses to maintain and modulate synaptic efficiency.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









Comment in
-
Neuroscience: Nanocolumns at the heart of the synapse.Nature. 2016 Aug 11;536(7615):151-2. doi: 10.1038/nature18917. Epub 2016 Jul 27. Nature. 2016. PMID: 27462816 No abstract available.
References
-
- Volk L, Chiu SL, Sharma K, Huganir RL. Glutamate Synapses in Human Cognitive Disorders. Annu Rev Neurosci. 2015;38:127–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008181/GM/NIGMS NIH HHS/United States
- F31 MH105105/MH/NIMH NIH HHS/United States
- F30-MH102891/MH/NIMH NIH HHS/United States
- NS090644/NS/NINDS NIH HHS/United States
- R01-MH080046/MH/NIMH NIH HHS/United States
- F30-MH105111/MH/NIMH NIH HHS/United States
- F30 MH102891/MH/NIMH NIH HHS/United States
- F31-MH105105/MH/NIMH NIH HHS/United States
- F30 MH105111/MH/NIMH NIH HHS/United States
- R01 GM106000/GM/NIGMS NIH HHS/United States
- T32-GM008181/GM/NIGMS NIH HHS/United States
- S10 OD010352/OD/NIH HHS/United States
- R01 NS090644/NS/NINDS NIH HHS/United States
- R37 MH080046/MH/NIMH NIH HHS/United States
- R01 MH080046/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

