Structure of the adenosine A(2A) receptor bound to an engineered G protein
- PMID: 27462812
- PMCID: PMC4979997
- DOI: 10.1038/nature18966
Structure of the adenosine A(2A) receptor bound to an engineered G protein
Erratum in
-
Erratum: Structure of the adenosine A2A receptor bound to an engineered G protein.Nature. 2016 Oct 27;538(7626):542. doi: 10.1038/nature19803. Epub 2016 Sep 14. Nature. 2016. PMID: 27629518 No abstract available.
Abstract
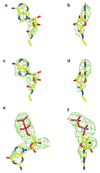
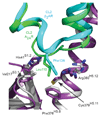
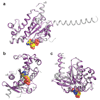
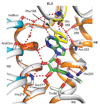
G-protein-coupled receptors (GPCRs) are essential components of the signalling network throughout the body. To understand the molecular mechanism of G-protein-mediated signalling, solved structures of receptors in inactive conformations and in the active conformation coupled to a G protein are necessary. Here we present the structure of the adenosine A(2A) receptor (A(2A)R) bound to an engineered G protein, mini-Gs, at 3.4 Å resolution. Mini-Gs binds to A(2A)R through an extensive interface (1,048 Å2) that is similar, but not identical, to the interface between Gs and the β2-adrenergic receptor. The transition of the receptor from an agonist-bound active-intermediate state to an active G-protein-bound state is characterized by a 14 Å shift of the cytoplasmic end of transmembrane helix 6 (H6) away from the receptor core, slight changes in the positions of the cytoplasmic ends of H5 and H7 and rotamer changes of the amino acid side chains Arg3.50, Tyr5.58 and Tyr7.53. There are no substantial differences in the extracellular half of the receptor around the ligand binding pocket. The A(2A)R-mini-Gs structure highlights both the diversity and similarity in G-protein coupling to GPCRs and hints at the potential complexity of the molecular basis for G-protein specificity.
Figures








References
-
- Venkatakrishnan AJ, et al. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

