A novel excitatory network for the control of breathing
- PMID: 27462817
- PMCID: PMC5479418
- DOI: 10.1038/nature18944
A novel excitatory network for the control of breathing
Abstract
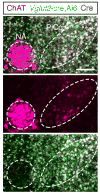
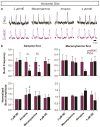
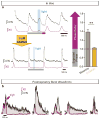
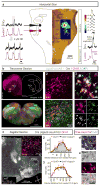
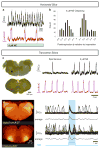
Breathing must be tightly coordinated with other behaviours such as vocalization, swallowing, and coughing. These behaviours occur after inspiration, during a respiratory phase termed postinspiration. Failure to coordinate postinspiration with inspiration can result in aspiration pneumonia, the leading cause of death in Alzheimer's disease, Parkinson's disease, dementia, and other neurodegenerative diseases. Here we describe an excitatory network that generates the neuronal correlate of postinspiratory activity in mice. Glutamatergic-cholinergic neurons form the basis of this network, and GABA (γ-aminobutyric acid)-mediated inhibition establishes the timing and coordination relative to inspiration. We refer to this network as the postinspiratory complex (PiCo). The PiCo has autonomous rhythm-generating properties and is necessary and sufficient for postinspiratory activity in vivo.The PiCo also shows distinct responses to neuromodulators when compared to other excitatory brainstem networks. On the basis of the discovery of the PiCo, we propose that each of the three phases of breathing is generated by a distinct excitatory network: the pre-Bötzinger complex, which has been linked to inspiration; the PiCo, as described here for the neuronal control of postinspiration; and the lateral parafacial region (pF(L)), which has been associated with active expiration, a respiratory phase that is recruited during high metabolic demand.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

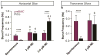







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

