Identification of a Monoclonal Antibody That Attenuates Antiphospholipid Syndrome-Related Pregnancy Complications and Thrombosis
- PMID: 27463336
- PMCID: PMC4963039
- DOI: 10.1371/journal.pone.0158757
Identification of a Monoclonal Antibody That Attenuates Antiphospholipid Syndrome-Related Pregnancy Complications and Thrombosis
Abstract
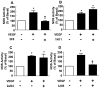
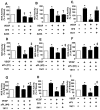
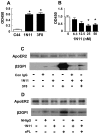
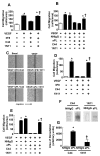
In the antiphospholipid syndrome (APS), patients produce antiphospholipid antibodies (aPL) that promote thrombosis and adverse pregnancy outcomes. Current therapy with anticoagulation is only partially effective and associated with multiple complications. We previously discovered that aPL recognition of cell surface β2-glycoprotein I (β2-GPI) initiates apolipoprotein E receptor 2 (apoER2)-dependent signaling in endothelial cells and in placental trophoblasts that ultimately promotes thrombosis and fetal loss, respectively. Here we sought to identify a monoclonal antibody (mAb) to β2-GPI that negates aPL-induced processes in cell culture and APS disease endpoints in mice. In a screen measuring endothelial NO synthase (eNOS) activity in cultured endothelial cells, we found that whereas aPL inhibit eNOS, the mAb 1N11 does not, and instead 1N11 prevents aPL action. Coimmunoprecipitation studies revealed that 1N11 decreases pathogenic antibody binding to β2-GPI, and it blocks aPL-induced complex formation between β2-GPI and apoER2. 1N11 also prevents aPL antagonism of endothelial cell migration, and in mice it reverses the impairment in reendothelialization caused by aPL, which underlies the non-thrombotic vascular occlusion provoked by disease-causing antibodies. In addition, aPL inhibition of trophoblast proliferation and migration is negated by 1N11, and the more than 6-fold increase in fetal resorption caused by aPL in pregnant mice is prevented by 1N11. Furthermore, the promotion of thrombosis by aPL is negated by 1N11. Thus, 1N11 has been identified as an mAb that attenuates APS-related pregnancy complications and thrombosis in mice. 1N11 may provide an efficacious, mechanism-based therapy to combat the often devastating conditions suffered by APS patients.
Conflict of interest statement
Figures
References
-
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb.Haemost. 2006;4: 295–306. - PubMed
-
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N.Engl.J Med. 2002;346: 752–763. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

