Effect of a Mobile Web App on Kidney Transplant Candidates' Knowledge About Increased Risk Donor Kidneys: A Randomized Controlled Trial
- PMID: 27463536
- PMCID: PMC5233689
- DOI: 10.1097/TP.0000000000001273
Effect of a Mobile Web App on Kidney Transplant Candidates' Knowledge About Increased Risk Donor Kidneys: A Randomized Controlled Trial
Abstract
Background: Kidney transplant candidates (KTCs) must provide informed consent to accept kidneys from increased risk donors (IRD), but poorly understand them. We conducted a multisite, randomized controlled trial to evaluate the efficacy of a mobile Web application, Inform Me, for increasing knowledge about IRDs.
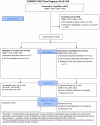
Methods: Kidney transplant candidates undergoing transplant evaluation at 2 transplant centers were randomized to use Inform Me after routine transplant education (intervention) or routine transplant education alone (control). Computer adaptive learning method reinforced learning by embedding educational material, and initial (test 1) and additional test questions (test 2) into each chapter. Knowledge (primary outcome) was assessed in person after education (tests 1 and 2), and 1 week later by telephone (test 3). Controls did not receive test 2. Willingness to accept an IRD kidney (secondary outcome) was assessed after tests 1 and 3. Linear regression test 1 knowledge scores were used to test the significance of Inform Me exposure after controlling for covariates. Multiple imputation was used for intention-to-treat analysis.
Results: Two hundred eighty-eight KTCs participated. Intervention participants had higher test 1 knowledge scores (mean difference, 6.61; 95% confidence interval [95% CI], 5.37-7.86) than control participants, representing a 44% higher score than control participants' scores. Intervention participants' knowledge scores increased with educational reinforcement (test 2) compared with control arm test 1 scores (mean difference, 9.50; 95% CI, 8.27-10.73). After 1 week, intervention participants' knowledge remained greater than controls' knowledge (mean difference, 3.63; 95% CI, 2.49-4.78) (test 3). Willingness to accept an IRD kidney did not differ between study arms at tests 1 and 3.
Conclusions: Inform Me use was associated with greater KTC knowledge about IRD kidneys above routine transplant education alone.
Trial registration: ClinicalTrials.gov NCT01859884.
References
-
- Ison M, Ho B, Leventhal J, Ladner D. Utilization and Characteristics of Kidney Donors in the US by OPTN-Defined Increased Risk Donor Status. Am J Transplant. 2015;15(suppl 3)
-
- Ison M, Abecassis M, Ladner D. Utilization and Characteristics of Liver Donors in the US by OPTN-Defined Increased Risk Donor Status. [abstract] http://www.atcmeetingabstracts.com/abstract/utilization-and-characterist.... Published 2015. Accessed May 2, 2015.
-
- Organ Procurement and Transplant Network (OPTN) Policies 1.2 and 2.4. Policy 2: Minimum Procurement Standards For An Organ Procurement Organization (OPO); Policy 4: Acquired Immune Deficiency Syndrome (AIDS) and Human Pituitary Derived Growth Hormone. http://www.unos.org/policiesandbylaws/policies.asp?resources]true. Updated 2016. Accessed August 24, 2009.
-
- Kucirka L, Namuyinga R, Hanrahan C, Montgomery R, Segev D. Formal policies and special informed consent are associated with higher provider utilization of CDC high-risk donor organs. Am J Transplant. 2009;9(3):629–635. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical