Non-Ligand-Induced Dimerization is Sufficient to Initiate the Signalling and Endocytosis of EGF Receptor
- PMID: 27463710
- PMCID: PMC5000598
- DOI: 10.3390/ijms17081200
Non-Ligand-Induced Dimerization is Sufficient to Initiate the Signalling and Endocytosis of EGF Receptor
Abstract
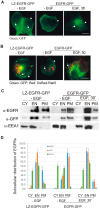
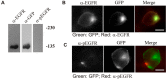
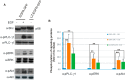
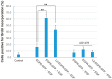
The binding of epidermal growth factor (EGF) to EGF receptor (EGFR) stimulates cell mitogenesis and survival through various signalling cascades. EGF also stimulates rapid EGFR endocytosis and its eventual degradation in lysosomes. The immediate events induced by ligand binding include receptor dimerization, activation of intrinsic tyrosine kinase and autophosphorylation. However, in spite of intensified efforts, the results regarding the roles of these events in EGFR signalling and internalization is still very controversial. In this study, we constructed a chimeric EGFR by replacing its extracellular domain with leucine zipper (LZ) and tagged a green fluorescent protein (GFP) at its C-terminus. We showed that the chimeric LZ-EGFR-GFP was constitutively dimerized. The LZ-EGFR-GFP dimer autophosphorylated each of its five well-defined C-terminal tyrosine residues as the ligand-induced EGFR dimer does. Phosphorylated LZ-EGFR-GFP was localized to both the plasma membrane and endosomes, suggesting it is capable of endocytosis. We also showed that LZ-EGFR-GFP activated major signalling proteins including Src homology collagen-like (Shc), extracellular signal-regulated kinase (ERK) and Akt. Moreover, LZ-EGFR-GFP was able to stimulate cell proliferation. These results indicate that non-ligand induced dimerization is sufficient to activate EGFR and initiate cell signalling and EGFR endocytosis. We conclude that receptor dimerization is a critical event in EGF-induced cell signalling and EGFR endocytosis.
Keywords: EGF receptors; dimerization; endocytosis; leucine zipper; signal transduction.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

