Biotin Protein Ligase Is a Target for New Antibacterials
- PMID: 27463729
- PMCID: PMC5039522
- DOI: 10.3390/antibiotics5030026
Biotin Protein Ligase Is a Target for New Antibacterials
Abstract
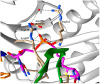
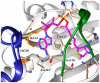
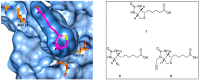
There is a desperate need for novel antibiotic classes to combat the rise of drug resistant pathogenic bacteria, such as Staphylococcus aureus. Inhibitors of the essential metabolic enzyme biotin protein ligase (BPL) represent a promising drug target for new antibacterials. Structural and biochemical studies on the BPL from S. aureus have paved the way for the design and development of new antibacterial chemotherapeutics. BPL employs an ordered ligand binding mechanism for the synthesis of the reaction intermediate biotinyl-5'-AMP from substrates biotin and ATP. Here we review the structure and catalytic mechanism of the target enzyme, along with an overview of chemical analogues of biotin and biotinyl-5'-AMP as BPL inhibitors reported to date. Of particular promise are studies to replace the labile phosphoroanhydride linker present in biotinyl-5'-AMP with alternative bioisosteres. A novel in situ click approach using a mutant of S. aureus BPL as a template for the synthesis of triazole-based inhibitors is also presented. These approaches can be widely applied to BPLs from other bacteria, as well as other closely related metabolic enzymes and antibacterial drug targets.
Keywords: Staphylococcus aureus; X-ray crystallography; antibiotic; biotin; biotin protein ligase; in situ click chemistry; inhibitor design.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures









Similar articles
-
Advanced Resistance Studies Identify Two Discrete Mechanisms in Staphylococcus aureus to Overcome Antibacterial Compounds that Target Biotin Protein Ligase.Antibiotics (Basel). 2020 Apr 6;9(4):165. doi: 10.3390/antibiotics9040165. Antibiotics (Basel). 2020. PMID: 32268615 Free PMC article.
-
Structure guided design of biotin protein ligase inhibitors for antibiotic discovery.Curr Top Med Chem. 2014;14(1):4-20. doi: 10.2174/1568026613666131111103149. Curr Top Med Chem. 2014. PMID: 24236729 Review.
-
Biotin analogues with antibacterial activity are potent inhibitors of biotin protein ligase.ACS Med Chem Lett. 2012 May 23;3(6):509-14. doi: 10.1021/ml300106p. eCollection 2012 Jun 14. ACS Med Chem Lett. 2012. PMID: 24900501 Free PMC article.
-
Selective inhibition of biotin protein ligase from Staphylococcus aureus.J Biol Chem. 2012 May 18;287(21):17823-17832. doi: 10.1074/jbc.M112.356576. Epub 2012 Mar 21. J Biol Chem. 2012. PMID: 22437830 Free PMC article.
-
Mechanisms Governing Precise Protein Biotinylation.Trends Biochem Sci. 2017 May;42(5):383-394. doi: 10.1016/j.tibs.2017.02.001. Epub 2017 Mar 3. Trends Biochem Sci. 2017. PMID: 28268045 Review.
Cited by
-
Proximity Dependent Biotinylation: Key Enzymes and Adaptation to Proteomics Approaches.Mol Cell Proteomics. 2020 May;19(5):757-773. doi: 10.1074/mcp.R120.001941. Epub 2020 Mar 3. Mol Cell Proteomics. 2020. PMID: 32127388 Free PMC article. Review.
-
A Canonical Biotin Synthesis Enzyme, 8-Amino-7-Oxononanoate Synthase (BioF), Utilizes Different Acyl Chain Donors in Bacillus subtilis and Escherichia coli.Appl Environ Microbiol. 2017 Dec 15;84(1):e02084-17. doi: 10.1128/AEM.02084-17. Print 2018 Jan 1. Appl Environ Microbiol. 2017. PMID: 29054876 Free PMC article.
-
Advanced Resistance Studies Identify Two Discrete Mechanisms in Staphylococcus aureus to Overcome Antibacterial Compounds that Target Biotin Protein Ligase.Antibiotics (Basel). 2020 Apr 6;9(4):165. doi: 10.3390/antibiotics9040165. Antibiotics (Basel). 2020. PMID: 32268615 Free PMC article.
-
New Series of BPL Inhibitors To Probe the Ribose-Binding Pocket of Staphylococcus aureus Biotin Protein Ligase.ACS Med Chem Lett. 2016 Oct 10;7(12):1068-1072. doi: 10.1021/acsmedchemlett.6b00248. eCollection 2016 Dec 8. ACS Med Chem Lett. 2016. PMID: 27994739 Free PMC article.
-
Bacteria exposed to antiviral drugs develop antibiotic cross-resistance and unique resistance profiles.Commun Biol. 2023 Aug 12;6(1):837. doi: 10.1038/s42003-023-05177-3. Commun Biol. 2023. PMID: 37573457 Free PMC article.
References
-
- Ferguson J. Healthcare-associated methicillin-resistant Staph aureus (MRSA) control in Australia and New Zealand—2007 Australasian Society for Infectious Diseases (ASID) conference forum convened by healthcare infection control special interest group (HICSIG) Healthc. Infect. 2007;12:60–66. doi: 10.1071/HI07060. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

