KSHV-Mediated Regulation of Par3 and SNAIL Contributes to B-Cell Proliferation
- PMID: 27463802
- PMCID: PMC4963126
- DOI: 10.1371/journal.ppat.1005801
KSHV-Mediated Regulation of Par3 and SNAIL Contributes to B-Cell Proliferation
Expression of concern in
-
Expression of Concern: KSHV-Mediated Regulation of Par3 and SNAIL Contributes to B-Cell Proliferation.PLoS Pathog. 2022 Apr 11;18(4):e1010480. doi: 10.1371/journal.ppat.1010480. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35404957 Free PMC article. No abstract available.
Abstract
Studies have suggested that Epithelial-Mesenchymal Transition (EMT) and transformation is an important step in progression to cancer. Par3 (partitioning-defective protein) is a crucial factor in regulating epithelial cell polarity. However, the mechanism by which the latency associated nuclear antigen (LANA) encoded by Kaposi's Sarcoma associated herpesvirus (KSHV) regulates Par3 and EMTs markers (Epithelial-Mesenchymal Transition) during viral-mediated B-cell oncogenesis has not been fully explored. Moreover, several studies have demonstrated a crucial role for EMT markers during B-cell malignancies. In this study, we demonstrate that Par3 is significantly up-regulated in KSHV-infected primary B-cells. Further, Par3 interacted with LANA in KSHV positive and LANA expressing cells which led to translocation of Par3 from the cell periphery to a predominantly nuclear signal. Par3 knockdown led to reduced cell proliferation and increased apoptotic induction. Levels of SNAIL was elevated, and E-cadherin was reduced in the presence of LANA or Par3. Interestingly, KSHV infection in primary B-cells led to enhancement of SNAIL and down-regulation of E-cadherin in a temporal manner. Importantly, knockdown of SNAIL, a major EMT regulator, in KSHV cells resulted in reduced expression of LANA, Par3, and enhanced E-cadherin. Also, SNAIL bound to the promoter region of p21 and can regulate its activity. Further a SNAIL inhibitor diminished NF-kB signaling through upregulation of Caspase3 in KSHV positive cells in vitro. This was also supported by upregulation of SNAIL and Par3 in BC-3 transplanted NOD-SCID mice which has potential as a therapeutic target for KSHV-associated B-cell lymphomas.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
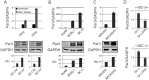

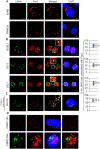


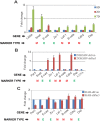
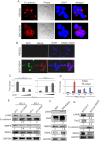



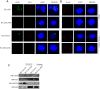
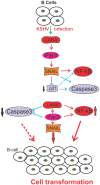
Similar articles
-
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen and angiogenin interact with common host proteins, including annexin A2, which is essential for survival of latently infected cells.J Virol. 2012 Feb;86(3):1589-607. doi: 10.1128/JVI.05754-11. Epub 2011 Nov 30. J Virol. 2012. PMID: 22130534 Free PMC article.
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with multifunctional angiogenin to utilize its antiapoptotic functions.J Virol. 2012 Jun;86(11):5974-91. doi: 10.1128/JVI.00070-12. Epub 2012 Mar 21. J Virol. 2012. PMID: 22438557 Free PMC article.
-
Viral latent proteins as targets for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma.Curr Drug Targets Infect Disord. 2003 Jun;3(2):129-35. doi: 10.2174/1568005033481150. Curr Drug Targets Infect Disord. 2003. PMID: 12769790 Review.
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
Cited by
-
KSHV-encoded vCyclin can modulate HIF1α levels to promote DNA replication in hypoxia.Elife. 2021 Jul 19;10:e57436. doi: 10.7554/eLife.57436. Elife. 2021. PMID: 34279223 Free PMC article.
-
Methylation of KSHV vCyclin by PRMT5 contributes to cell cycle progression and cell proliferation.PLoS Pathog. 2024 Sep 10;20(9):e1012535. doi: 10.1371/journal.ppat.1012535. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39255317 Free PMC article.
-
Expression of Concern: KSHV-Mediated Regulation of Par3 and SNAIL Contributes to B-Cell Proliferation.PLoS Pathog. 2022 Apr 11;18(4):e1010480. doi: 10.1371/journal.ppat.1010480. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35404957 Free PMC article. No abstract available.
-
The polarity protein PARD3 and cancer.Oncogene. 2021 Jun;40(25):4245-4262. doi: 10.1038/s41388-021-01813-6. Epub 2021 Jun 7. Oncogene. 2021. PMID: 34099863 Review.
-
Cellular responses to human cytomegalovirus infection: Induction of a mesenchymal-to-epithelial transition (MET) phenotype.Proc Natl Acad Sci U S A. 2017 Sep 26;114(39):E8244-E8253. doi: 10.1073/pnas.1710799114. Epub 2017 Sep 5. Proc Natl Acad Sci U S A. 2017. PMID: 28874566 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865–1869. - PubMed
-
- McAllister SC, Moses AV (2007) Endothelial cell- and lymphocyte-based in vitro systems for understanding KSHV biology. Curr Top Microbiol Immunol 312: 211–244. - PubMed
-
- Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, et al. (1996) Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88: 645–656. - PubMed
-
- Chen YB, Rahemtullah A, Hochberg E (2007) Primary effusion lymphoma. Oncologist 12: 569–576. - PubMed
-
- Elgui de Oliveira D (2007) DNA viruses in human cancer: an integrated overview on fundamental mechanisms of viral carcinogenesis. Cancer Lett 247: 182–196. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

