Long-term evolution of multiple sclerosis disability in the treatment era
- PMID: 27464262
- PMCID: PMC5105678
- DOI: 10.1002/ana.24747
Long-term evolution of multiple sclerosis disability in the treatment era
Abstract
Objective: To characterize the accrual of long-term disability in a cohort of actively treated multiple sclerosis (MS) patients and to assess whether clinical and magnetic resonance imaging (MRI) data used in clinical trials have long-term prognostic value.
Methods: This is a prospective study of 517 actively managed MS patients enrolled at a single center.
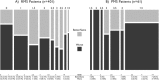
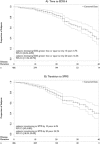
Results: More than 91% of patients were retained, with data ascertained up to 10 years after the baseline visit. At this last assessment, neurologic disability as measured by the Expanded Disability Status Scale (EDSS) was stable or improved compared to baseline in 41% of patients. Subjects with no evidence of disease activity (NEDA) by clinical and MRI criteria during the first 2 years had long-term outcomes that were no different from those of the cohort as a whole. 25-OH vitamin D serum levels were inversely associated with short-term MS disease activity; however, these levels had no association with long-term disability. At a median time of 16.8 years after disease onset, 10.7% (95% confidence interval [CI] = 7.2-14%) of patients reached an EDSS ≥ 6, and 18.1% (95% CI = 13.5-22.5%) evolved from relapsing MS to secondary progressive MS (SPMS).
Interpretation: Rates of worsening and evolution to SPMS were substantially lower when compared to earlier natural history studies. Notably, the NEDA 2-year endpoint was not a predictor of long-term stability. Finally, the data call into question the utility of annual MRI assessments as a treat-to-target approach for MS care. Ann Neurol 2016;80:499-510.
© 2016 The Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest Companies that make MS DMTs described in this manuscript include: Bayer, Biogen, EMD Serono, Pfizer, and Teva. The following authors disclosed financial relationships with these companies. SB: consultancy- EMD Serono, Teva; BACC: consultancy- Biogen, EMD Serono, Teva; GRC: consultancy- Biogen, Teva, EMD Serono, Pfizer; DSG: speaking fees- EMD Serono, Teva; EC-H: consultancy- Biogen, Teva; DTO: consultancy and speaking fees- Teva; DP: consultancy- Biogen; SSZ: speaking fees- Biogen.
Figures
References
-
- Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–1438. - PubMed
-
- Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770–782. - PubMed
-
- Tremlett H, Paty D, Devonshire V. The natural history of primary progressive MS in British Columbia, Canada. Neurology 2005;65:1919–1923. - PubMed
-
- Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006;66:172–177. - PubMed
-
- Debouverie M, Pittion‐Vouyovitch S, Louis S, et al. Natural history of multiple sclerosis in a population‐based cohort. Eur J Neurol 2008;15:916–921. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

