Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions
- PMID: 27464306
- PMCID: PMC5429324
- DOI: 10.1111/bph.13545
Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions
Abstract
Background and purpose: The diterpenoids carnosol (CS) and carnosic acid (CA) from Salvia spp. exert prominent anti-inflammatory activities but their molecular mechanisms remained unclear. Here we investigated the effectiveness of CS and CA in inflammatory pain and the cellular interference with their putative molecular targets.
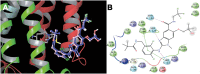
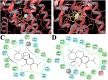
Experimental approach: The effects of CS and CA in different models of inflammatory pain were investigated. The inhibition of key enzymes in eicosanoid biosynthesis, namely microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LO) was confirmed by CS and CA, and we determined the consequence on the eicosanoid network in activated human primary monocytes and neutrophils. Molecular interactions and binding modes of CS and CA to target enzymes were analyzed by docking studies.
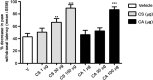
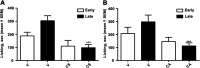
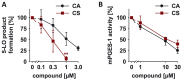
Key results: CS and CA displayed significant and dose-dependent anti-inflammatory and anti-nociceptive effects in carrageenan-induced mouse hyperalgesia 4 h post injection of the stimuli, and also inhibited the analgesic response in the late phase of the formalin test. Moreover, both compounds potently inhibited cell-free mPGES-1 and 5-LO activity and preferentially suppressed the formation of mPGES-1 and 5-LO-derived products in cellular studies. Our in silico analysis for mPGES-1 and 5-LO supports that CS and CA are dual 5-LO/mPGES-1 inhibitors.
Conclusion and implications: In summary, we propose that the combined inhibition of mPGES-1 and 5-LO by CS and CA essentially contributes to the bioactivity of these diterpenoids. Our findings pave the way for a rational use of Salvia spp., traditionally used as anti-inflammatory remedy, in the continuous expanding context of nutraceuticals.
Linked articles: This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc.
© 2016 The British Pharmacological Society.
Figures


Similar articles
-
Potent inhibition of human 5-lipoxygenase and microsomal prostaglandin E₂ synthase-1 by the anti-carcinogenic and anti-inflammatory agent embelin.Biochem Pharmacol. 2013 Aug 15;86(4):476-86. doi: 10.1016/j.bcp.2013.04.015. Epub 2013 Apr 24. Biochem Pharmacol. 2013. PMID: 23623753
-
Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1.J Pharmacol Exp Ther. 2012 Jul;342(1):169-76. doi: 10.1124/jpet.112.193847. Epub 2012 Apr 16. J Pharmacol Exp Ther. 2012. PMID: 22511203 Free PMC article.
-
Lipophilic extracts of Leucas zeylanica, a multi-purpose medicinal plant in the tropics, inhibit key enzymes involved in inflammation and gout.J Ethnopharmacol. 2018 Oct 5;224:474-481. doi: 10.1016/j.jep.2018.04.042. Epub 2018 May 1. J Ethnopharmacol. 2018. PMID: 29727733
-
Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis.Biotechnol Adv. 2018 Nov 1;36(6):1709-1723. doi: 10.1016/j.biotechadv.2018.02.010. Epub 2018 Feb 15. Biotechnol Adv. 2018. PMID: 29454981 Review.
-
Computer-Aided Drug Design of Anti-inflammatory Agents Targeting Microsomal Prostaglandin E2 Synthase-1 (mPGES-1).Curr Med Chem. 2022;29(33):5397-5419. doi: 10.2174/0929867329666220317122948. Curr Med Chem. 2022. PMID: 35301943 Review.
Cited by
-
Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief.Molecules. 2021 Dec 16;26(24):7632. doi: 10.3390/molecules26247632. Molecules. 2021. PMID: 34946714 Free PMC article. Review.
-
An Integrated Proteomics and Bioinformatics Approach Reveals the Anti-inflammatory Mechanism of Carnosic Acid.Front Pharmacol. 2018 Apr 16;9:370. doi: 10.3389/fphar.2018.00370. eCollection 2018. Front Pharmacol. 2018. PMID: 29713284 Free PMC article.
-
Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases.Molecules. 2020 Dec 18;25(24):6016. doi: 10.3390/molecules25246016. Molecules. 2020. PMID: 33353211 Free PMC article. Review.
-
In Silico, In Vitro, and In Vivo Analysis of Tanshinone IIA and Cryptotanshinone from Salvia miltiorrhiza as Modulators of Cyclooxygenase-2/mPGES-1/Endothelial Prostaglandin EP3 Pathway.Biomolecules. 2022 Jan 7;12(1):99. doi: 10.3390/biom12010099. Biomolecules. 2022. PMID: 35053247 Free PMC article.
-
Carnosic Acid Induces Anti-Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Involving a Crosstalk Between the Nrf2/HO-1 Axis and NF-κB.Mol Neurobiol. 2018 Jan;55(1):890-897. doi: 10.1007/s12035-017-0389-6. Epub 2017 Jan 12. Mol Neurobiol. 2018. PMID: 28083817
References
-
- Amici R, Bigogno C, Boggio R, Colombo A, Courtney SM, Dal Zuffo R et al. (2014). Chiral resolution and pharmacological characterization of the enantiomers of the Hsp90 inhibitor 2‐amino‐7‐[4‐fluoro‐2‐(3‐pyridyl)phenyl]‐4‐methyl‐7,8‐dihydro‐6H‐quinazolin‐5‐one oxime. ChemMedChem 9: 1574–1585. - PubMed
-
- Bruna S, Giovannini A, De Benedetti L, Principato MC, Ruffoni B (2006). Molecular analysis of Salvia spp. through RAPID markers. Acta Hort 723 (Proceedings of the Ist International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation, 2006): 157–160.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

