Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions
- PMID: 27464306
- PMCID: PMC5429324
- DOI: 10.1111/bph.13545
Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions
Abstract
Background and purpose: The diterpenoids carnosol (CS) and carnosic acid (CA) from Salvia spp. exert prominent anti-inflammatory activities but their molecular mechanisms remained unclear. Here we investigated the effectiveness of CS and CA in inflammatory pain and the cellular interference with their putative molecular targets.
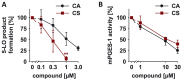
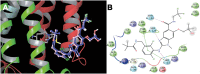
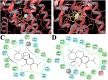
Experimental approach: The effects of CS and CA in different models of inflammatory pain were investigated. The inhibition of key enzymes in eicosanoid biosynthesis, namely microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LO) was confirmed by CS and CA, and we determined the consequence on the eicosanoid network in activated human primary monocytes and neutrophils. Molecular interactions and binding modes of CS and CA to target enzymes were analyzed by docking studies.
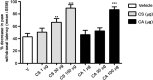
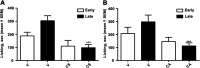
Key results: CS and CA displayed significant and dose-dependent anti-inflammatory and anti-nociceptive effects in carrageenan-induced mouse hyperalgesia 4 h post injection of the stimuli, and also inhibited the analgesic response in the late phase of the formalin test. Moreover, both compounds potently inhibited cell-free mPGES-1 and 5-LO activity and preferentially suppressed the formation of mPGES-1 and 5-LO-derived products in cellular studies. Our in silico analysis for mPGES-1 and 5-LO supports that CS and CA are dual 5-LO/mPGES-1 inhibitors.
Conclusion and implications: In summary, we propose that the combined inhibition of mPGES-1 and 5-LO by CS and CA essentially contributes to the bioactivity of these diterpenoids. Our findings pave the way for a rational use of Salvia spp., traditionally used as anti-inflammatory remedy, in the continuous expanding context of nutraceuticals.
Linked articles: This article is part of a themed section on Principles of Pharmacological Research of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.11/issuetoc.
© 2016 The British Pharmacological Society.
Figures


References
-
- Amici R, Bigogno C, Boggio R, Colombo A, Courtney SM, Dal Zuffo R et al. (2014). Chiral resolution and pharmacological characterization of the enantiomers of the Hsp90 inhibitor 2‐amino‐7‐[4‐fluoro‐2‐(3‐pyridyl)phenyl]‐4‐methyl‐7,8‐dihydro‐6H‐quinazolin‐5‐one oxime. ChemMedChem 9: 1574–1585. - PubMed
-
- Bauer J, Kuehnl S, Rollinger JM, Scherer O, Northoff H, Stuppner H et al. (2012). Carnosol and carnosic acids from <styled-content style="fixed-case"><styled-content style="italic-in-any-context">Salvia officinalis</styled-content></styled-content> inhibit microsomal prostaglandin E2 synthase‐1. J Pharmacol Exp Ther 342: 169–176. - PMC - PubMed
-
- Bruna S, Giovannini A, De Benedetti L, Principato MC, Ruffoni B (2006). Molecular analysis of Salvia spp. through RAPID markers. Acta Hort 723 (Proceedings of the Ist International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation, 2006): 157–160.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

