Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline
- PMID: 27464347
- PMCID: PMC5489232
- DOI: 10.1002/mus.25267
Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline
Abstract
Introduction: Age-associated muscle strength decline is a major contributing factor to increased late-life functional decline and comorbidity, and is strongly associated with early mortality. Although all parts of the neuromuscular system seem to be affected by aging, dying-back of motor axons likely plays a major role.
Methods: We compared the degeneration in ventral roots and neuromuscular junction denervation in young and aged mice and correlated the findings with strength and electrophysiological measures.
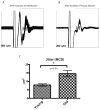
Results: With normal aging, there is little decline in motor axon numbers in the ventral roots, but the neuromuscular junctions show marked partial denervation that is associated with increased jitter on stimulated single fiber electromyography and a decrease in muscle strength.
Conclusions: These findings suggest that dying-back axonal degeneration may be partially responsible for the electrophysiological and strength changes observed with aging. Muscle Nerve 55: 894-901, 2017.
Keywords: SFEMG; denervation; distal axon; dying-back axonopathy; frailty; neuromuscular junction.
© 2016 Wiley Periodicals, Inc.
Figures



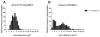
References
-
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. - PubMed
-
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

