Prelamin A impairs 53BP1 nuclear entry by mislocalizing NUP153 and disrupting the Ran gradient
- PMID: 27464478
- PMCID: PMC5114580
- DOI: 10.1111/acel.12506
Prelamin A impairs 53BP1 nuclear entry by mislocalizing NUP153 and disrupting the Ran gradient
Abstract
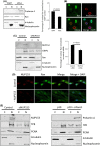
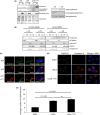
The nuclear lamina is essential for the proper structure and organization of the nucleus. Deregulation of A-type lamins can compromise genomic stability, alter chromatin organization and cause premature vascular aging. Here, we show that accumulation of the lamin A precursor, prelamin A, inhibits 53BP1 recruitment to sites of DNA damage and increases basal levels of DNA damage in aged vascular smooth muscle cells. We identify that this genome instability arises through defective nuclear import of 53BP1 as a consequence of abnormal topological arrangement of nucleoporin NUP153. We show for the first time that this nucleoporin is important for the nuclear localization of Ran and that the deregulated Ran gradient is likely to be compromising the nuclear import of 53BP1. Importantly, many of the defects associated with prelamin A expression were significantly reduced upon treatment with Remodelin, a small molecule recently reported to reverse deficiencies associated with abnormal nuclear lamina.
Keywords: 53BP1; NUP153; Ran gradient; cytoplasmic-nuclear trafficking; prelamin A; vascular disease.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




Similar articles
-
The role of prelamin A post-translational maturation in stress response and 53BP1 recruitment.Front Cell Dev Biol. 2022 Nov 16;10:1018102. doi: 10.3389/fcell.2022.1018102. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467410 Free PMC article.
-
Nucleoporin NUP153 guards genome integrity by promoting nuclear import of 53BP1.Cell Death Differ. 2012 May;19(5):798-807. doi: 10.1038/cdd.2011.150. Epub 2011 Nov 11. Cell Death Differ. 2012. PMID: 22075984 Free PMC article.
-
Localisation of Nup153 and SENP1 to nuclear pore complexes is required for 53BP1-mediated DNA double-strand break repair.J Cell Sci. 2017 Jul 15;130(14):2306-2316. doi: 10.1242/jcs.198390. Epub 2017 Jun 2. J Cell Sci. 2017. PMID: 28576968
-
Defective DNA-damage repair induced by nuclear lamina dysfunction is a key mediator of smooth muscle cell aging.Biochem Soc Trans. 2011 Dec;39(6):1780-5. doi: 10.1042/BST20110703. Biochem Soc Trans. 2011. PMID: 22103525 Review.
-
Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells.Biochem Soc Trans. 2011 Dec;39(6):1698-704. doi: 10.1042/BST20110657. Biochem Soc Trans. 2011. PMID: 22103510 Review.
Cited by
-
Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome.Nutrients. 2019 Jun 1;11(6):1251. doi: 10.3390/nu11061251. Nutrients. 2019. PMID: 31159371 Free PMC article. Review.
-
Towards delineating the chain of events that cause premature senescence in the accelerated aging syndrome Hutchinson-Gilford progeria (HGPS).Biochem Soc Trans. 2020 Jun 30;48(3):981-991. doi: 10.1042/BST20190882. Biochem Soc Trans. 2020. PMID: 32539085 Free PMC article. Review.
-
Mineral Stress Drives Loss of Heterochromatin: An Early Harbinger of Vascular Inflammaging and Calcification.Circ Res. 2025 Feb 14;136(4):379-399. doi: 10.1161/CIRCRESAHA.124.325374. Epub 2025 Jan 22. Circ Res. 2025. PMID: 39840455 Free PMC article.
-
Advances in the understanding of nuclear pore complexes in human diseases.J Cancer Res Clin Oncol. 2024 Jul 30;150(7):374. doi: 10.1007/s00432-024-05881-5. J Cancer Res Clin Oncol. 2024. PMID: 39080077 Free PMC article. Review.
-
A nuclear lamina-chromatin-Ran GTPase axis modulates nuclear import and DNA damage signaling.Aging Cell. 2019 Feb;18(1):e12851. doi: 10.1111/acel.12851. Epub 2018 Dec 19. Aging Cell. 2019. PMID: 30565836 Free PMC article.
References
-
- Al‐Haboubi T, Shumaker DK, Koser J, Wehnert M, Fahrenkrog B (2011) Distinct association of the nuclear pore protein Nup153 with A‐ and B‐type lamins. Nucleus 2, 500–509. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

