Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations
- PMID: 27465185
- PMCID: PMC5845841
- DOI: 10.1097/PRS.0000000000002356
Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations
Abstract
Background: Optimization of fat grafting continues to gain increasing attention in the field of regenerative medicine. "Nanofat grafting" implements mechanical emulsification and injection of standard lipoaspirate for the correction of superficial rhytides and skin discoloration; however, little is known about the cellular constituents of the graft. Based on recent evidence that various stressors can induce progenitor activity, the authors hypothesized that the shear forces used in common fat grafting techniques may impact their regenerative capacities.
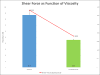
Methods: Lipoaspirates were obtained from 10 patients undergoing elective procedures. Half of each sample was subjected to nanofat processing; the other half was left unchallenged. The viscosity of each sample was measured for computational analysis. The stromal vascular fraction of each sample was isolated, quantified, and analyzed by means of flow cytometry with two multicolor fluorescence antibody panels.
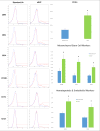
Results: Standard lipoaspirate is ideally suited for mechanical stress induction. The mechanical emulsification involved in nanofat processing did not affect cell number; however, viability was greatly reduced when compared with the stromal vascular fraction of standard lipoaspirate. Interestingly, nanofat processing resulted in stress-induced stromal vascular fraction with a higher proportion of endothelial progenitor cells, mesenchymal stem cells, and multilineage differentiating stress-enduring cells. Single-parameter analysis also revealed significant increases in CD34, CD13, CD73, and CD146 of the stress-induced stromal vascular fraction, markers associated with mesenchymal stem cell activity.
Conclusions: Mechanical processing used in techniques such as nanofat grafting induces the up-regulation of progenitor phenotypes consistent with multipotency and pluripotency. These data provide a first step in characterizing the potential regenerative benefits realized through stress induction in fat grafting.
Clincal question/level of evidence: Therapeutic, V.
Conflict of interest statement
There are no other relevant financial disclosures.
Figures




Comment in
-
Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations.Plast Reconstr Surg. 2017 Apr;139(4):1024e-1025e. doi: 10.1097/PRS.0000000000003202. Plast Reconstr Surg. 2017. PMID: 28002249 No abstract available.
References
-
- Gutierrez-Fernandez M, Otero-Ortega L, Ramos-Cejudo J, Rodriguez-Frutos B, Fuentes B, Diez-Tejedor E. Adipose tissue-derived mesenchymal stem cells as a strategy to improve recovery after stroke. Expert Opin Biol Ther. 2015;15(6):873–881. - PubMed
-
- Richardson SM, Kalamegam G, Pushparaj PN, et al. Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods. 2015 - PubMed
-
- Kolle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382(9898):1113–1120. - PubMed
-
- Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue engineering. 2006;12(12):3375–3382. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

