Allele-specific locus binding and genome editing by CRISPR at the p16INK4a locus
- PMID: 27465215
- PMCID: PMC4964623
- DOI: 10.1038/srep30485
Allele-specific locus binding and genome editing by CRISPR at the p16INK4a locus
Abstract
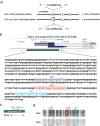
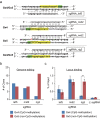
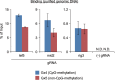
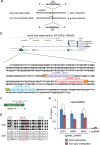
The clustered regularly interspaced short palindromic repeats (CRISPR) system has been adopted for a wide range of biological applications including genome editing. In some cases, dissection of genome functions requires allele-specific genome editing, but the use of CRISPR for this purpose has not been studied in detail. In this study, using the p16INK4a gene in HCT116 as a model locus, we investigated whether chromatin states, such as CpG methylation, or a single-nucleotide gap form in a target site can be exploited for allele-specific locus binding and genome editing by CRISPR in vivo. First, we showed that allele-specific locus binding and genome editing could be achieved by targeting allele-specific CpG-methylated regions, which was successful for one, but not all guide RNAs. In this regard, molecular basis underlying the success remains elusive at this stage. Next, we demonstrated that an allele-specific single-nucleotide gap form could be employed for allele-specific locus binding and genome editing by CRISPR, although it was important to avoid CRISPR tolerance of a single nucleotide mismatch brought about by mismatched base skipping. Our results provide information that might be useful for applications of CRISPR in studies of allele-specific functions in the genomes.
Conflict of interest statement
T.F. and H.F. have filed a patent on enChIP (“Method for isolating specific genomic regions using DNA-binding molecules recognizing endogenous DNA sequences,” patent number WO2014/125668).
Figures

Similar articles
-
Postnatal Cardiac Gene Editing Using CRISPR/Cas9 With AAV9-Mediated Delivery of Short Guide RNAs Results in Mosaic Gene Disruption.Circ Res. 2017 Oct 27;121(10):1168-1181. doi: 10.1161/CIRCRESAHA.116.310370. Epub 2017 Aug 29. Circ Res. 2017. PMID: 28851809
-
Genome-wide determination of on-target and off-target characteristics for RNA-guided DNA methylation by dCas9 methyltransferases.Gigascience. 2018 Mar 1;7(3):1-19. doi: 10.1093/gigascience/giy011. Gigascience. 2018. PMID: 29635374 Free PMC article.
-
CRISPR Diagnosis and Therapeutics with Single Base Pair Precision.Trends Mol Med. 2020 Mar;26(3):337-350. doi: 10.1016/j.molmed.2019.09.008. Epub 2019 Nov 29. Trends Mol Med. 2020. PMID: 31791730 Review.
-
CRISPR Guide RNA Design Guidelines for Efficient Genome Editing.Methods Mol Biol. 2020;2166:331-342. doi: 10.1007/978-1-0716-0712-1_19. Methods Mol Biol. 2020. PMID: 32710418
-
Recent advances in the CRISPR genome editing tool set.Exp Mol Med. 2019 Nov 5;51(11):1-11. doi: 10.1038/s12276-019-0339-7. Exp Mol Med. 2019. PMID: 31685795 Free PMC article. Review.
Cited by
-
IL-3-Induced Immediate Expression of c-fos and c-jun Is Modulated by the IKK2-JNK Axis.Cells. 2022 Apr 25;11(9):1451. doi: 10.3390/cells11091451. Cells. 2022. PMID: 35563758 Free PMC article.
-
Trichostatin A for Efficient CRISPR-Cas9 Gene Editing of Human Pluripotent Stem Cells.CRISPR J. 2023 Oct;6(5):473-485. doi: 10.1089/crispr.2023.0033. Epub 2023 Sep 7. CRISPR J. 2023. PMID: 37676985 Free PMC article.
-
Radioresistance, DNA Damage and DNA Repair in Cells With Moderate Overexpression of RPA1.Front Genet. 2020 Jul 31;11:855. doi: 10.3389/fgene.2020.00855. eCollection 2020. Front Genet. 2020. PMID: 32849834 Free PMC article.
-
A stem cell marker KLF5 regulates CCAT1 via three-dimensional genome structure in colorectal cancer cells.Br J Cancer. 2022 Jan;126(1):109-119. doi: 10.1038/s41416-021-01579-4. Epub 2021 Oct 27. Br J Cancer. 2022. PMID: 34707247 Free PMC article.
-
Detection of genome-edited cells by oligoribonucleotide interference-PCR.DNA Res. 2018 Aug 1;25(4):395-407. doi: 10.1093/dnares/dsy012. DNA Res. 2018. PMID: 29718217 Free PMC article.
References
-
- Sun N. & Zhao H. Transcription activator-like effector nucleases (TALENs): A highly efficient and versatile tool for genome editing. Biotechnol. Bioeng. 110, 1811–1821 (2013). - PubMed
-
- Doudna J. A. & Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

