Melanoma dormancy in a mouse model is linked to GILZ/FOXO3A-dependent quiescence of disseminated stem-like cells
- PMID: 27465291
- PMCID: PMC4964333
- DOI: 10.1038/srep30405
Melanoma dormancy in a mouse model is linked to GILZ/FOXO3A-dependent quiescence of disseminated stem-like cells
Abstract
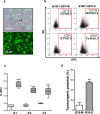
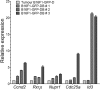
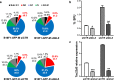
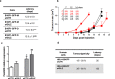
Metastatic cancer relapses following the reactivation of dormant, disseminated tumour cells; however, the cells and factors involved in this reactivation are just beginning to be identified. Using an immunotherapy-based syngeneic model of melanoma dormancy and GFP-labelled dormant cell-derived cell lines, we determined that vaccination against melanoma prevented tumour growth but did not prevent tumour cell dissemination or eliminate all tumour cells. The persistent disseminated melanoma tumour cells were quiescent and asymptomatic for one year. The quiescence/activation of these cells in vitro and the dormancy of melanoma in vivo appeared to be regulated by glucocorticoid-induced leucine zipper (GILZ)-mediated immunosuppression. GILZ expression was low in dormant cell-derived cultures, and re-expression of GILZ inactivated FOXO3A and its downstream target, p21CIP1. The ability of dormancy-competent cells to re-enter the cell cycle increased after a second round of cellular dormancy in vivo in association with shortened tumour dormancy period and faster and more aggressive melanoma relapse. Our data indicate that future cancer treatments should be adjusted according to the stage of disease progression.
Figures

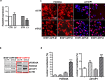
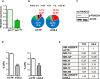
References
-
- Kleffel S. & Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv. Exp. Med. Biol. 734, 145–179 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

