Urothelial purine release during filling of murine and primate bladders
- PMID: 27465992
- PMCID: PMC5243211
- DOI: 10.1152/ajprenal.00387.2016
Urothelial purine release during filling of murine and primate bladders
Abstract
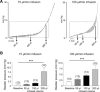
During urinary bladder filling the bladder urothelium releases chemical mediators that in turn transmit information to the nervous and muscular systems to regulate sensory sensation and detrusor muscle activity. Defects in release of urothelial mediators may cause bladder dysfunctions that are characterized with aberrant bladder sensation during bladder filling. Previous studies have demonstrated release of ATP from the bladder urothelium during bladder filling, and ATP remains the most studied purine mediator that is released from the urothelium. However, the micturition cycle is likely regulated by multiple purine mediators, since various purine receptors are found present in many cell types in the bladder wall, including urothelial cells, afferent nerves, interstitial cells in lamina propria, and detrusor smooth muscle cells. Information about the release of other biologically active purines during bladder filling is still lacking. Decentralized bladders from C57BL/6 mice and Cynomolgus monkeys (Macaca fascicularis) were filled with physiological solution at different rates. Intraluminal fluid was analyzed by high-performance liquid chromatography with fluorescence detection for simultaneous evaluation of ATP, ADP, AMP, adenosine, nicotinamide adenine dinucleotide (NAD+), ADP-ribose, and cADP-ribose content. We also measured ex vivo bladder filling pressures and performed cystometry in conscious unrestrained mice at different filling rates. ATP, ADP, AMP, NAD+, ADPR, cADPR, and adenosine were detected released intravesically at different ratios during bladder filling. Purine release increased with increased volumes and rates of filling. Our results support the concept that multiple urothelium-derived purines likely contribute to the complex regulation of bladder sensation during bladder filling.
Keywords: adenosine 5′-triphosphate release; nicotinamide adenine dinucleotide release; urothelium.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Andersson KE. Purinergic signalling in the urinary bladder. Auton Neurosci 191: 78–81, 2015. - PubMed
-
- Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007. - PubMed
-
- Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-performance liquid chromatographic technique for detection of a fluorescent analogue of ADP-ribose in isolated blood vessel preparations. Analytical Biochemistry 305: 269–276, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

