The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity
- PMID: 27466192
- PMCID: PMC5291228
- DOI: 10.1093/hmg/ddw232
The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity
Abstract
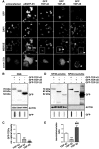
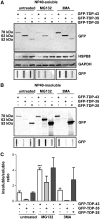
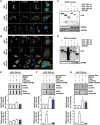
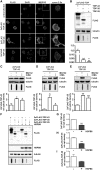
Aggregation of TAR-DNA-binding protein 43 (TDP-43) and of its fragments TDP-25 and TDP-35 occurs in amyotrophic lateral sclerosis (ALS). TDP-25 and TDP-35 act as seeds for TDP-43 aggregation, altering its function and exerting toxicity. Thus, inhibition of TDP-25 and TDP-35 aggregation and promotion of their degradation may protect against cellular damage. Upregulation of HSPB8 is one possible approach for this purpose, since this chaperone promotes the clearance of an ALS associated fragments of TDP-43 and is upregulated in the surviving motor neurones of transgenic ALS mice and human patients. We report that overexpression of HSPB8 in immortalized motor neurones decreased the accumulation of TDP-25 and TDP-35 and that protection against mislocalized/truncated TDP-43 was observed for HSPB8 in Drosophila melanogaster Overexpression of HSP67Bc, the functional ortholog of human HSPB8, suppressed the eye degeneration caused by the cytoplasmic accumulation of a TDP-43 variant with a mutation in the nuclear localization signal (TDP-43-NLS). TDP-43-NLS accumulation in retinal cells was counteracted by HSP67Bc overexpression. According with this finding, downregulation of HSP67Bc increased eye degeneration, an effect that is consistent with the accumulation of high molecular weight TDP-43 species and ubiquitinated proteins. Moreover, we report a novel Drosophila model expressing TDP-35, and show that while TDP-43 and TDP-25 expression in the fly eyes causes a mild degeneration, TDP-35 expression leads to severe neurodegeneration as revealed by pupae lethality; the latter effect could be rescued by HSP67Bc overexpression. Collectively, our data demonstrate that HSPB8 upregulation mitigates TDP-43 fragment mediated toxicity, in mammalian neuronal cells and flies.
© The Author 2016. Published by Oxford University Press.
Figures
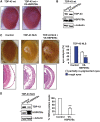
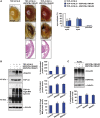
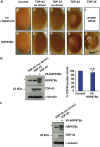
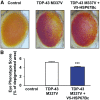
References
-
- Robberecht W., Philips T. (2013) The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci., 14, 248–264. - PubMed
-
- Strong M.J., Yang W. (2011) The frontotemporal syndromes of ALS. Clinicopathological correlates. J. Mol. Neurosci.: MN, 45, 648–655. - PubMed
-
- Haapasalo A., Viswanathan J., Bertram L., Soininen H., Tanzi R.E., Hiltunen M. (2010) Emerging role of Alzheimer's disease-associated ubiquilin-1 in protein aggregation. Biochem. Soc. Trans., 38, 150–155. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

