Maternal Gametophyte Effects on Seed Development in Maize
- PMID: 27466227
- PMCID: PMC5012389
- DOI: 10.1534/genetics.116.191833
Maternal Gametophyte Effects on Seed Development in Maize
Abstract
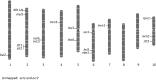
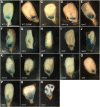
Flowering plants, like placental mammals, have an extensive maternal contribution toward progeny development. Plants are distinguished from animals by a genetically active haploid phase of growth and development between meiosis and fertilization, called the gametophyte. Flowering plants are further distinguished by the process of double fertilization that produces sister progeny, the endosperm and the embryo, of the seed. Because of this, there is substantial gene expression in the female gametophyte that contributes to the regulation of growth and development of the seed. A primary function of the endosperm is to provide growth support to its sister embryo. Several mutations in Zea mays subsp. mays have been identified that affect the contribution of the mother gametophyte to the seed. The majority affect both the endosperm and the embryo, although some embryo-specific effects have been observed. Many alter the pattern of expression of a marker for the basal endosperm transfer layer, a tissue that transports nutrients from the mother plant to the developing seed. Many of them cause abnormal development of the female gametophyte prior to fertilization, revealing potential cellular mechanisms of maternal control of seed development. These effects include reduced central cell size, abnormal architecture of the central cell, abnormal numbers and morphology of the antipodal cells, and abnormal egg cell morphology. These mutants provide insight into the logic of seed development, including necessary features of the gametes and supporting cells prior to fertilization, and set up future studies on the mechanisms regulating maternal contributions to the seed.
Keywords: embryo sac; endosperm transfer layer; gametophyte; maize; maternal effect.
Copyright © 2016 by the Genetics Society of America.
Figures



Similar articles
-
Maternal regulation of seed growth and patterning in flowering plants.Curr Top Dev Biol. 2020;140:257-282. doi: 10.1016/bs.ctdb.2019.10.008. Epub 2019 Nov 19. Curr Top Dev Biol. 2020. PMID: 32591076
-
Analysis of stunter1, a maize mutant with reduced gametophyte size and maternal effects on seed development.Genetics. 2011 Apr;187(4):1085-97. doi: 10.1534/genetics.110.125286. Epub 2011 Jan 26. Genetics. 2011. PMID: 21270392 Free PMC article.
-
Genetic analysis of female gametophyte development and function.Plant Cell. 1998 Jan;10(1):5-17. doi: 10.1105/tpc.10.1.5. Plant Cell. 1998. PMID: 9477569 Free PMC article. Review.
-
Antipodal cells persist through fertilization in the female gametophyte of Arabidopsis.Plant Reprod. 2014 Dec;27(4):197-203. doi: 10.1007/s00497-014-0251-1. Epub 2014 Nov 12. Plant Reprod. 2014. PMID: 25389024
-
The long and winding road: transport pathways for amino acids in Arabidopsis seeds.Plant Reprod. 2018 Sep;31(3):253-261. doi: 10.1007/s00497-018-0334-5. Epub 2018 Mar 16. Plant Reprod. 2018. PMID: 29549431 Review.
Cited by
-
Quantitative Trait Loci for Seed Size Variation in Cucurbits - A Review.Front Plant Sci. 2020 Mar 20;11:304. doi: 10.3389/fpls.2020.00304. eCollection 2020. Front Plant Sci. 2020. PMID: 32265957 Free PMC article. Review.
-
Genetic Screens to Target Embryo and Endosperm Pathways in Arabidopsis and Maize.Methods Mol Biol. 2020;2122:3-14. doi: 10.1007/978-1-0716-0342-0_1. Methods Mol Biol. 2020. PMID: 31975291
-
Bacteria existing in pre-pollinated styles (silks) can defend the exposed male gamete fertilization channel of maize against an environmental Fusarium pathogen.Front Plant Sci. 2023 Dec 4;14:1292109. doi: 10.3389/fpls.2023.1292109. eCollection 2023. Front Plant Sci. 2023. PMID: 38111882 Free PMC article.
-
A Rapid Pipeline for Pollen- and Anther-Specific Gene Discovery Based on Transcriptome Profiling Analysis of Maize Tissues.Int J Mol Sci. 2021 Jun 26;22(13):6877. doi: 10.3390/ijms22136877. Int J Mol Sci. 2021. PMID: 34206810 Free PMC article.
-
Selection on the gametophyte: Modeling alternation of generations in plants.Appl Plant Sci. 2022 Apr 21;10(2):e11472. doi: 10.1002/aps3.11472. eCollection 2022 Mar-Apr. Appl Plant Sci. 2022. PMID: 35495198 Free PMC article.
References
-
- Autran D., Baroux C., Raissig M. T., Lenormand T., Wittig M., et al. , 2011. Maternal epigenetic pathways control parental contributions to Arabidopsis early embryogenesis. Cell 145: 707–719. - PubMed
-
- Baroux C., Grossniklaus U., 2015. The Maternal-to-Zygotic Transition in Flowering Plants: Evidence, Mechanisms, and Plasticity. Curr. Top. Dev. Biol. 113: 351–371. - PubMed
-
- Baroux C., Blanvillain R., Gallois P., 2001. Paternally inherited transgenes are down-regulated but retain low activity during early embryogenesis in Arabidopsis. FEBS Lett. 509: 11–16. - PubMed
-
- Barrell P. J., Grossniklaus U., 2005. Confocal microscopy of whole ovules for analysis of reproductive development: the elongate1 mutant affects meiosis II. Plant J. 43: 309–320. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

