Discordant relationship between Essure microinsert position and tubal occlusion
- PMID: 27466315
- PMCID: PMC4964221
- DOI: 10.1136/bcr-2016-216535
Discordant relationship between Essure microinsert position and tubal occlusion
Abstract
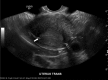
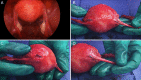
Hysteroscopic sterilisation with Essure requires confirmation of tubal occlusion by hysterosalpingogram or microinsert position by transvaginal sonography 3 months after placement before women can rely on the method for pregnancy prevention. A 39-year-old woman underwent hysteroscopic sterilisation via Essure, with successful bilateral tubal occlusion documented on hysterosalpingogram. She had a subsequent unintended pregnancy and termination, and presented with persistent pelvic pain and other non-specific symptoms. She underwent a laparoscopic-assisted vaginal hysterectomy with bilateral salpingectomy, with complete resolution of her symptoms. Pathological evaluation demonstrated a perforated Essure microinsert and ipsilateral tubal occlusion, and a correctly placed Essure microinsert with ipsilateral tubal patency. Clinicians should be cautious about the assumption that correctly placed microinserts based on ultrasonography, hysterosalpingogram or laparoscopic evaluation assures occlusion success.
2016 BMJ Publishing Group Ltd.
Figures

Similar articles
-
Technique for the Laparoscopic Removal of Essure Microinserts.J Minim Invasive Gynecol. 2016 May-Jun;23(4):472. doi: 10.1016/j.jmig.2016.01.005. Epub 2016 Jan 8. J Minim Invasive Gynecol. 2016. PMID: 26776673
-
Use of Hysterosalpingo-Foam Sonography for Assessment of the Efficacy of Essure Hysteroscopic Sterilization.J Ultrasound Med. 2018 Aug;37(8):1929-1935. doi: 10.1002/jum.14539. Epub 2018 Jan 18. J Ultrasound Med. 2018. PMID: 29344973
-
One-step transvaginal three-dimensional hysterosalpingo-foam sonography (3D-HyFoSy) confirmation test for Essure® follow-up: a multicenter study.Ultrasound Obstet Gynecol. 2018 Jan;51(1):134-141. doi: 10.1002/uog.17398. Ultrasound Obstet Gynecol. 2018. PMID: 28067009
-
Hysteroscopic tubal sterilization: an evidence-based analysis.Ont Health Technol Assess Ser. 2013 Oct 1;13(21):1-35. eCollection 2013. Ont Health Technol Assess Ser. 2013. PMID: 24228084 Free PMC article. Review.
-
Review of Sterilization Techniques and Clinical Updates.J Minim Invasive Gynecol. 2018 Nov-Dec;25(7):1157-1164. doi: 10.1016/j.jmig.2017.09.012. Epub 2017 Sep 20. J Minim Invasive Gynecol. 2018. PMID: 28939482 Review.
References
-
- Camlin C, Escandon I. Sterilization incidence and prevalence. In: Landry E, ed. Contraceptive sterilization: global issues and trends. New York, NY: EngenderHealth, 2002:16–64.
-
- Ahlborg J, Cordero C, Cullins V et al. . Female sterilization. In: Landry E, ed. Contraceptive sterilization: global issues and trends. New York, NY: EngenderHealth, 2002:139–60.
-
- Bayer HealthCare Pharmaceuticals. Essure permanent birth control: Instructions for use. Milpitas, CA: Bayer Healthcare LLC, 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
