Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism
- PMID: 27466319
- PMCID: PMC5042579
- DOI: 10.1091/mbc.E16-01-0029
Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism
Abstract
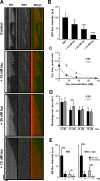
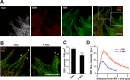
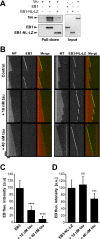
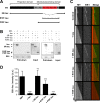
Proper regulation of microtubule dynamics is essential for cell functions and involves various microtubule-associated proteins (MAPs). Among them, end-binding proteins (EBs) accumulate at microtubule plus ends, whereas structural MAPs bind along the microtubule lattice. Recent data indicate that the structural MAP tau modulates EB subcellular localization in neurons. However, the molecular determinants of EB/tau interaction remain unknown, as is the effect of this interplay on microtubule dynamics. Here we investigate the mechanisms governing EB/tau interaction in cell-free systems and cellular models. We find that tau inhibits EB tracking at microtubule ends. Tau and EBs form a complex via the C-terminal region of EBs and the microtubule-binding sites of tau. These two domains are required for the inhibitory activity of tau on EB localization to microtubule ends. Moreover, the phosphomimetic mutation S262E within tau microtubule-binding sites impairs EB/tau interaction and prevents the inhibitory effect of tau on EB comets. We further show that microtubule dynamic parameters vary, depending on the combined activities of EBs and tau proteins. Overall our results demonstrate that tau directly antagonizes EB function through a phosphorylation-dependent mechanism. This study highlights a novel role for tau in EB regulation, which might be impaired in neurodegenerative disorders.
© 2016 Ramirez-Rios et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

Similar articles
-
A TIRF microscopy assay to decode how tau regulates EB's tracking at microtubule ends.Methods Cell Biol. 2017;141:179-197. doi: 10.1016/bs.mcb.2017.06.013. Epub 2017 Jul 17. Methods Cell Biol. 2017. PMID: 28882301
-
Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells.J Neurochem. 2015 Jun;133(5):653-67. doi: 10.1111/jnc.13091. Epub 2015 Apr 8. J Neurochem. 2015. PMID: 25761518
-
Adenomatous Polyposis Coli as a Scaffold for Microtubule End-Binding Proteins.J Mol Biol. 2019 May 3;431(10):1993-2005. doi: 10.1016/j.jmb.2019.03.028. Epub 2019 Apr 6. J Mol Biol. 2019. PMID: 30959051
-
Regulation of EB1/3 proteins by classical MAPs in neurons.Bioarchitecture. 2014 Jan-Feb;4(1):1-5. doi: 10.4161/bioa.27774. Epub 2014 Jan 10. Bioarchitecture. 2014. PMID: 24452057 Free PMC article. Review.
-
Quantitative analysis of MAP-mediated regulation of microtubule dynamic instability in vitro focus on Tau.Methods Cell Biol. 2010;95:481-503. doi: 10.1016/S0091-679X(10)95024-3. Methods Cell Biol. 2010. PMID: 20466149 Review.
Cited by
-
Repositioning Microtubule Stabilizing Drugs for Brain Disorders.Front Cell Neurosci. 2018 Aug 8;12:226. doi: 10.3389/fncel.2018.00226. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30135644 Free PMC article. Review.
-
ADNP, a Microtubule Interacting Protein, Provides Neuroprotection Through End Binding Proteins and Tau: An Amplifier Effect.Front Mol Neurosci. 2018 May 1;11:151. doi: 10.3389/fnmol.2018.00151. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29765303 Free PMC article. No abstract available.
-
Cellular cartography: Towards an atlas of the neuronal microtubule cytoskeleton.Front Cell Dev Biol. 2023 Mar 22;11:1052245. doi: 10.3389/fcell.2023.1052245. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37035244 Free PMC article. Review.
-
ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: a novel avenue for protection against tauopathy.Mol Psychiatry. 2017 Sep;22(9):1335-1344. doi: 10.1038/mp.2016.255. Epub 2017 Jan 24. Mol Psychiatry. 2017. PMID: 28115743
-
Prolonged depletion of profilin 1 or F-actin causes an adaptive response in microtubules.J Cell Biol. 2024 Jul 1;223(7):e202309097. doi: 10.1083/jcb.202309097. Epub 2024 May 9. J Cell Biol. 2024. PMID: 38722279 Free PMC article.
References
-
- Ackmann M, Wiech H, Mandelkow E. Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem. 2000;275:30335–30343. - PubMed
-
- Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–3419. - PubMed
-
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–298. - PubMed
-
- Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 2007;450:1100–1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

