Neural Activity in the Ventral Pallidum Encodes Variation in the Incentive Value of a Reward Cue
- PMID: 27466340
- PMCID: PMC4961780
- DOI: 10.1523/JNEUROSCI.0736-16.2016
Neural Activity in the Ventral Pallidum Encodes Variation in the Incentive Value of a Reward Cue
Abstract
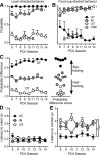
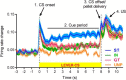
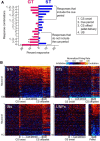
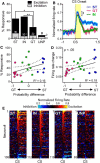
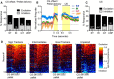
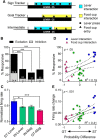
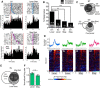
There is considerable individual variation in the extent to which reward cues are attributed with incentive salience. For example, a food-predictive conditioned stimulus (CS; an illuminated lever) becomes attractive, eliciting approach toward it only in some rats ("sign trackers," STs), whereas others ("goal trackers," GTs) approach the food cup during the CS period. The purpose of this study was to determine how individual differences in Pavlovian approach responses are represented in neural firing patterns in the major output structure of the mesolimbic system, the ventral pallidum (VP). Single-unit in vivo electrophysiology was used to record neural activity in the caudal VP during the performance of ST and GT conditioned responses. All rats showed neural responses to both cue onset and reward delivery but, during the CS period, STs showed greater neural activity than GTs both in terms of the percentage of responsive neurons and the magnitude of the change in neural activity. Furthermore, neural activity was positively correlated with the degree of attraction to the cue. Given that the CS had equal predictive value in STs and GTs, we conclude that neural activity in the VP largely reflects the degree to which the CS was attributed with incentive salience.
Significance statement: Cues associated with reward can acquire motivational properties (i.e., incentive salience) that cause them to have a powerful influence on desire and motivated behavior. There are individual differences in sensitivity to reward-paired cues, with some individuals attaching greater motivational value to cues than others. Here, we investigated the neural activity associated with these individual differences in incentive salience. We found that cue-evoked neural firing in the ventral pallidum (VP) reflected the strength of incentive motivation, with the greatest neural responses occurring in individuals that demonstrated the strongest attraction to the cue. This suggests that the VP plays an important role in the process by which cues gain control over motivation and behavior.
Keywords: Pavlovian conditioning; goal tracking; motivation; rats; sign tracking; ventral pallidum.
Copyright © 2016 the authors 0270-6474/16/367957-14$15.00/0.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
