Reducing Endogenous α-Synuclein Mitigates the Degeneration of Selective Neuronal Populations in an Alzheimer's Disease Transgenic Mouse Model
- PMID: 27466341
- PMCID: PMC4961781
- DOI: 10.1523/JNEUROSCI.0775-16.2016
Reducing Endogenous α-Synuclein Mitigates the Degeneration of Selective Neuronal Populations in an Alzheimer's Disease Transgenic Mouse Model
Abstract
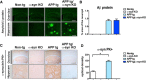
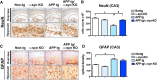
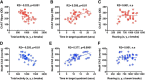
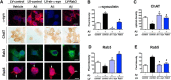
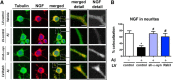
Alzheimer's disease (AD) is characterized by the progressive accumulation of amyloid β (Aβ) and microtubule associate protein tau, leading to the selective degeneration of neurons in the neocortex, limbic system, and nucleus basalis, among others. Recent studies have shown that α-synuclein (α-syn) also accumulates in the brains of patients with AD and interacts with Aβ and tau, forming toxic hetero-oligomers. Although the involvement of α-syn has been investigated extensively in Lewy body disease, less is known about the role of this synaptic protein in AD. Here, we found that reducing endogenous α-syn in an APP transgenic mouse model of AD prevented the degeneration of cholinergic neurons, ameliorated corresponding deficits, and recovered the levels of Rab3a and Rab5 proteins involved in intracellular transport and sorting of nerve growth factor and brain-derived neurotrophic factor. Together, these results suggest that α-syn might participate in mechanisms of vulnerability of selected neuronal populations in AD and that reducing α-syn might be a potential approach to protecting these populations from the toxic effects of Aβ.
Significance statement: Reducing endogenous α-synuclein (α-syn) in an APP transgenic mouse model of Alzheimer's disease (AD) prevented the degeneration of cholinergic neurons, ameliorated corresponding deficits, and recovered the levels of Rab3a and Rab5 proteins involved in intracellular transport and sorting of nerve growth factor and brain-derived neurotrophic factor. These results suggest that α-syn might participate in mechanisms of vulnerability of selected neuronal populations in AD and that reducing α-syn might be a potential approach to protecting these populations from the toxic effects of amyloid β.
Keywords: Alzheimer's disease; amyloid β oligomer; cholinergic neuron; selective vulnerability; transgenic animal model; α-synuclein.
Copyright © 2016 the authors 0270-6474/16/367971-14$15.00/0.
Figures






References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. - DOI - PubMed
-
- Bachhuber T, Katzmarski N, McCarter JF, Loreth D, Tahirovic S, Kamp F, Abou-Ajram C, Nuscher B, Serrano-Pozo A, Müller A, Prinz M, Steiner H, Hyman BT, Haass C, Meyer-Luehmann M. Inhibition of amyloid-beta plaque formation by alpha-synuclein. Nat Med. 2015;21:802–807. doi: 10.1038/nm.3885. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
