Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine
- PMID: 27466345
- PMCID: PMC4961783
- DOI: 10.1523/JNEUROSCI.1038-16.2016
Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine
Abstract
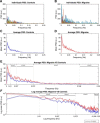
For many years, neurobiological theories have emphasized the importance of neuronal oscillations in the emergence of brain function. At the same time, clinical studies have shown that disturbances or irregularities in brain rhythms may relate to various common neurological conditions, including migraine. Increasing evidence suggests that the CNS plays a fundamental role in the predisposition to develop different forms of headache. Here, we present human imaging data that strongly support the presence of abnormal low-frequency oscillations (LFOs) in thalamocortical networks of patients in the interictal phase of migraine. Our results show that the main source of arrhythmic activity was localized to the higher-order thalamic relays of the medial dorsal nucleus. In addition, spontaneous LFOs in the thalamus were selectively associated with the headache attack frequency, meaning that the varying amplitude of dysrhythmia could predispose patients to recurrent attacks. Rhythmic cortical feedback to the thalamus is a major factor in the amplification of thalamocortical oscillations, making it a strong candidate for influencing neuronal excitability. We further speculate that the intrinsic dynamics of thalamocortical network oscillations are crucial for early sensory processing and therefore could underlie important pathophysiological processes involved in multisensory integration.
Significance statement: In many cases, migraine attacks are thought to begin centrally. A major obstacle to studying intrinsic brain activity has been the identification of the precise anatomical structures and functional networks that are involved in migraine. Here, we present imaging data that strongly support the presence of abnormal low-frequency oscillations in thalamocortical networks of patients in the interictal phase of migraine. This arrhythmic activity was localized to the higher-order thalamic relays of the medial dorsal nucleus and was selectively associated with headache attack frequency. Rhythmic cortical feedback to the thalamus is a major factor in the amplification of thalamocortical oscillations, making it a strong candidate for influencing neuronal excitability and higher-level processes involved in multisensory integration.
Keywords: frequency; headache; migraine; oscillations; pain; thalamocortical.
Copyright © 2016 the authors 0270-6474/16/368026-11$15.00/0.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
