DNA-Dye-Conjugates: Conformations and Spectra of Fluorescence Probes
- PMID: 27467071
- PMCID: PMC4965132
- DOI: 10.1371/journal.pone.0160229
DNA-Dye-Conjugates: Conformations and Spectra of Fluorescence Probes
Abstract
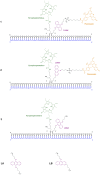
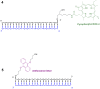
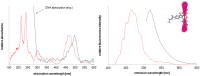
Extensive molecular-dynamics (MD) simulations have been used to investigate DNA-dye and DNA-photosensitizer conjugates, which act as reactants in templated reactions leading to the generation of fluorescent products in the presence of specific desoxyribonucleic acid sequences (targets). Such reactions are potentially suitable for detecting target nucleic acids in live cells by fluorescence microscopy or flow cytometry. The simulations show how the attached dyes/photosensitizers influence DNA structure and reveal the relative orientations of the chromophores with respect to each other. Our results will help to optimize the reactants for the templated reactions, especially length and structure of the spacers used to link reporter dyes or photosensitizers to the oligonucleotides responsible for target recognition. Furthermore, we demonstrate that the structural ensembles obtained from the simulations can be used to calculate steady-state UV-vis absorption and emission spectra. We also show how important quantities describing the quenching of the reporter dye via fluorescence resonance energy transfer (FRET) can be calculated from the simulation data, and we compare these for different relative chromophore geometries.
Conflict of interest statement
Figures

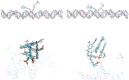


Similar articles
-
Computer simulation to investigate the FRET application in DNA hybridization systems.Phys Chem Chem Phys. 2011 Jun 7;13(21):10364-71. doi: 10.1039/c0cp02058c. Epub 2011 May 3. Phys Chem Chem Phys. 2011. PMID: 21537495
-
Two-step FRET as a structural tool.J Am Chem Soc. 2003 Jun 18;125(24):7336-43. doi: 10.1021/ja034564p. J Am Chem Soc. 2003. PMID: 12797808
-
Dispersion Correction Alleviates Dye Stacking of Single-Stranded DNA and RNA in Simulations of Single-Molecule Fluorescence Experiments.J Phys Chem B. 2018 Dec 13;122(49):11626-11639. doi: 10.1021/acs.jpcb.8b07537. Epub 2018 Oct 17. J Phys Chem B. 2018. PMID: 30285443
-
Distance determination in protein-DNA complexes using fluorescence resonance energy transfer.Methods Mol Biol. 2006;335:243-55. doi: 10.1385/1-59745-069-3:243. Methods Mol Biol. 2006. PMID: 16785632 Review.
-
Detecting RNA/DNA hybridization using double-labeled donor probes with enhanced fluorescence resonance energy transfer signals.Methods Mol Biol. 2006;335:43-56. doi: 10.1385/1-59745-069-3:43. Methods Mol Biol. 2006. PMID: 16785619 Review.
Cited by
-
Interaction of Thymine DNA Glycosylase with Oxidised 5-Methyl-cytosines in Their Amino- and Imino-Forms.Molecules. 2021 Sep 22;26(19):5728. doi: 10.3390/molecules26195728. Molecules. 2021. PMID: 34641273 Free PMC article.
-
DNA Transformations for Diagnosis and Therapy.Adv Funct Mater. 2021 Mar 17;31(12):2008279. doi: 10.1002/adfm.202008279. Epub 2020 Dec 27. Adv Funct Mater. 2021. PMID: 33613148 Free PMC article. Review.
-
New fluorogenic triacylglycerols as sensors for dynamic measurement of lipid oxidation.Anal Bioanal Chem. 2025 Jan;417(2):287-296. doi: 10.1007/s00216-024-05642-w. Epub 2024 Nov 21. Anal Bioanal Chem. 2025. PMID: 39570389 Free PMC article.
-
2'-Alkynyl spin-labelling is a minimally perturbing tool for DNA structural analysis.Nucleic Acids Res. 2020 Apr 6;48(6):2830-2840. doi: 10.1093/nar/gkaa086. Nucleic Acids Res. 2020. PMID: 32052020 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

