Variation in Human Cytochrome P-450 Drug-Metabolism Genes: A Gateway to the Understanding of Plasmodium vivax Relapses
- PMID: 27467145
- PMCID: PMC4965052
- DOI: 10.1371/journal.pone.0160172
Variation in Human Cytochrome P-450 Drug-Metabolism Genes: A Gateway to the Understanding of Plasmodium vivax Relapses
Erratum in
-
Correction: Variation in Human Cytochrome P-450 Drug-Metabolism Genes: A Gateway to the Understanding of Plasmodium vivax Relapses.PLoS One. 2018 Feb 1;13(2):e0192534. doi: 10.1371/journal.pone.0192534. eCollection 2018. PLoS One. 2018. PMID: 29389962 Free PMC article.
Abstract
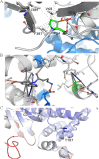
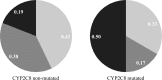
Although Plasmodium vivax relapses are classically associated with hypnozoite activation, it has been proposed that a proportion of these cases are due to primaquine (PQ) treatment failure caused by polymorphisms in cytochrome P-450 2D6 (CYP2D6). Here, we present evidence that CYP2D6 polymorphisms are implicated in PQ failure, which was reinforced by findings in genetically similar parasites, and may explain a number of vivax relapses. Using a computational approach, these polymorphisms were predicted to affect the activity of CYP2D6 through changes in the structural stability that could lead to disruption of the PQ-enzyme interactions. Furthermore, because PQ is co-administered with chloroquine (CQ), we investigated whether CQ-impaired metabolism by cytochrome P-450 2C8 (CYP2C8) could also contribute to vivax recurrences. Our results show that CYP2C8-mutated patients frequently relapsed early (<42 days) and had a higher proportion of genetically similar parasites, suggesting the possibility of recrudescence due to CQ therapeutic failure. These results highlight the importance of pharmacogenetic studies as a tool to monitor the efficacy of antimalarial therapy.
Conflict of interest statement
Figures

Similar articles
-
Novel Insights into Plasmodium vivax Therapeutic Failure: CYP2D6 Activity and Time of Exposure to Malaria Modulate the Risk of Recurrence.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02056-19. doi: 10.1128/AAC.02056-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32122891 Free PMC article.
-
Several Plasmodium vivax relapses after correct primaquine treatment in a patient with impaired cytochrome P450 2D6 function.Malar J. 2020 Jul 17;19(1):259. doi: 10.1186/s12936-020-03326-1. Malar J. 2020. PMID: 32680522 Free PMC article.
-
Association of Impaired Cytochrome P450 2D6 Activity Genotype and Phenotype With Therapeutic Efficacy of Primaquine Treatment for Latent Plasmodium vivax Malaria.JAMA Netw Open. 2018 Aug 3;1(4):e181449. doi: 10.1001/jamanetworkopen.2018.1449. JAMA Netw Open. 2018. PMID: 30646129 Free PMC article.
-
Primaquine treatment and relapse in Plasmodium vivax malaria.Pathog Glob Health. 2016;110(1):1-8. doi: 10.1080/20477724.2015.1133033. Epub 2016 Feb 18. Pathog Glob Health. 2016. PMID: 27077309 Free PMC article. Review.
-
Impact of CYP2D6 Genetic Variation on Radical Cure of Plasmodium vivax Malaria.Clin Pharmacol Ther. 2021 Sep;110(3):595-598. doi: 10.1002/cpt.2313. Epub 2021 Jun 20. Clin Pharmacol Ther. 2021. PMID: 34042179 Review.
Cited by
-
Influence of CYP2C8, CYP3A4, and CYP3A5 Host Genotypes on Early Recurrence of Plasmodium vivax.Antimicrob Agents Chemother. 2020 Jun 23;64(7):e02125-19. doi: 10.1128/AAC.02125-19. Print 2020 Jun 23. Antimicrob Agents Chemother. 2020. PMID: 32366712 Free PMC article.
-
Correction: Variation in Human Cytochrome P-450 Drug-Metabolism Genes: A Gateway to the Understanding of Plasmodium vivax Relapses.PLoS One. 2018 Feb 1;13(2):e0192534. doi: 10.1371/journal.pone.0192534. eCollection 2018. PLoS One. 2018. PMID: 29389962 Free PMC article.
-
CYP2D6 activity and the risk of recurrence of Plasmodium vivax malaria in the Brazilian Amazon: a prospective cohort study.Malar J. 2018 Feb 1;17(1):57. doi: 10.1186/s12936-017-2139-7. Malar J. 2018. PMID: 29390987 Free PMC article.
-
Pharmacogenomic variation in the Malagasy population: implications for the antimalarial drug primaquine metabolism.Pharmacogenomics. 2023 Jul;24(11):583-597. doi: 10.2217/pgs-2023-0091. Epub 2023 Aug 8. Pharmacogenomics. 2023. PMID: 37551613 Free PMC article.
-
SDM: a server for predicting effects of mutations on protein stability.Nucleic Acids Res. 2017 Jul 3;45(W1):W229-W235. doi: 10.1093/nar/gkx439. Nucleic Acids Res. 2017. PMID: 28525590 Free PMC article.
References
-
- Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhaes BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55(8):e67–74. - PubMed
-
- Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015;12(10):e1001891 10.1371/journal.pmed.1001891 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

