Immune-Modulation by Epidermal Growth Factor Receptor Inhibitors: Implication on Anti-Tumor Immunity in Lung Cancer
- PMID: 27467256
- PMCID: PMC4965069
- DOI: 10.1371/journal.pone.0160004
Immune-Modulation by Epidermal Growth Factor Receptor Inhibitors: Implication on Anti-Tumor Immunity in Lung Cancer
Abstract
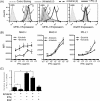
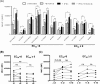
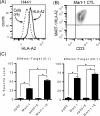
Skin toxicity is the most common toxicity caused by Epidermal Growth Factor Receptor (EGFR) inhibitors, and has been associated with clinical efficacy. As EGFR inhibitors enhance the expression of antigen presenting molecules in affected skin keratinocytes, they may concurrently facilitate neo-antigen presentation in lung cancer tumor cells contributing to anti-tumor immunity. Here, we investigated the modulatory effect of the EGFR inhibitor, erlotinib on antigen presenting molecules and PD-L1, prominent immune checkpoint protein, of skin keratinocytes and lung cancer cell lines to delineate the link between EGFR signaling pathway inhibition and potential anti-tumor immunity. Erlotinib up-regulated MHC-I and MHC-II proteins on IFNγ treated keratinocytes but abrogated IFNγ-induced expression of PD-L1, suggesting the potential role of infiltrating autoreactive T cells in the damage of keratinocytes in affected skin. Interestingly, the surface expression of MHC-I, MHC-II, and PD-L1 was up-regulated in response to IFNγ more often in lung cancer cell lines sensitive to erlotinib, but only expression of PD-L1 was inhibited by erlotinib. Further, erlotinib significantly increased T cell mediated cytotoxicity on lung cancer cells. Lastly, the analysis of gene expression dataset of 186 lung cancer cell lines from Cancer Cell Line Encyclopedia demonstrated that overexpression of PD-L1 was associated with sensitivity to erlotinib and higher expression of genes related to antigen presenting pathways and IFNγ signaling pathway. Our findings suggest that the EGFR inhibitors can facilitate anti-tumor adaptive immune responses by breaking tolerance especially in EGFR driven lung cancer that are associated with overexpression of PD-L1 and genes related to antigen presentation and inflammation.
Conflict of interest statement
Figures



References
-
- Howlader N, Noone AM, krapcho M (2014) SEER Cancer Statistics Review, 1975–2011. Available at http://seercancergov/csr/1975_2011.
-
- Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. (2015) Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 13: 515–524. - PubMed
-
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352: 786–792. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

