Allosteric Inhibition of Factor XIIIa. Non-Saccharide Glycosaminoglycan Mimetics, but Not Glycosaminoglycans, Exhibit Promising Inhibition Profile
- PMID: 27467511
- PMCID: PMC4965010
- DOI: 10.1371/journal.pone.0160189
Allosteric Inhibition of Factor XIIIa. Non-Saccharide Glycosaminoglycan Mimetics, but Not Glycosaminoglycans, Exhibit Promising Inhibition Profile
Abstract
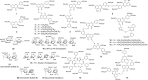
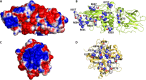
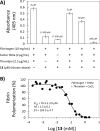
Factor XIIIa (FXIIIa) is a transglutaminase that catalyzes the last step in the coagulation process. Orthostery is the only approach that has been exploited to design FXIIIa inhibitors. Yet, allosteric inhibition of FXIIIa is a paradigm that may offer a key advantage of controlled inhibition over orthosteric inhibition. Such an approach is likely to lead to novel FXIIIa inhibitors that do not carry bleeding risks. We reasoned that targeting a collection of basic amino acid residues distant from FXIIIa's active site by using sulfated glycosaminoglycans (GAGs) or non-saccharide GAG mimetics (NSGMs) would lead to the discovery of the first allosteric FXIIIa inhibitors. We tested a library of 22 variably sulfated GAGs and NSGMs against human FXIIIa to discover promising hits. Interestingly, although some GAGs bound to FXIIIa better than NSGMs, no GAG displayed any inhibition. An undecasulfated quercetin analog was found to inhibit FXIIIa with reasonable potency (efficacy of 98%). Michaelis-Menten kinetic studies revealed an allosteric mechanism of inhibition. Fluorescence studies confirmed close correspondence between binding affinity and inhibition potency, as expected for an allosteric process. The inhibitor was reversible and at least 9-fold- and 26-fold selective over two GAG-binding proteins factor Xa (efficacy of 71%) and thrombin, respectively, and at least 27-fold selective over a cysteine protease papain. The inhibitor also inhibited the FXIIIa-mediated polymerization of fibrin in vitro. Overall, our work presents the proof-of-principle that FXIIIa can be allosterically modulated by sulfated non-saccharide agents much smaller than GAGs, which should enable the design of selective and safe anticoagulants.
Conflict of interest statement
Figures



Similar articles
-
Factor XIIIa inhibitors as potential novel drugs for venous thromboembolism.Eur J Med Chem. 2020 Aug 15;200:112442. doi: 10.1016/j.ejmech.2020.112442. Epub 2020 May 18. Eur J Med Chem. 2020. PMID: 32502864 Free PMC article. Review.
-
Ethacrynic acid is an inhibitor of human factor XIIIa.BMC Pharmacol Toxicol. 2022 Jun 1;23(1):35. doi: 10.1186/s40360-022-00575-5. BMC Pharmacol Toxicol. 2022. PMID: 35642005 Free PMC article.
-
Potent, Selective, Allosteric Inhibition of Human Plasmin by Sulfated Non-Saccharide Glycosaminoglycan Mimetics.J Med Chem. 2017 Jan 26;60(2):641-657. doi: 10.1021/acs.jmedchem.6b01474. Epub 2017 Jan 5. J Med Chem. 2017. PMID: 27976897 Free PMC article.
-
A molecular dynamics-based algorithm for evaluating the glycosaminoglycan mimicking potential of synthetic, homogenous, sulfated small molecules.PLoS One. 2017 Feb 9;12(2):e0171619. doi: 10.1371/journal.pone.0171619. eCollection 2017. PLoS One. 2017. PMID: 28182755 Free PMC article.
-
[Inhibitors of factor XIIIa].Hamostaseologie. 2002 Feb;22(1):43-7. Hamostaseologie. 2002. PMID: 12193984 Review. German.
Cited by
-
Proteolytic and nonproteolytic activation mechanisms result in conformationally and functionally different forms of coagulation factor XIII A.FEBS J. 2020 Feb;287(3):452-464. doi: 10.1111/febs.15040. Epub 2019 Aug 28. FEBS J. 2020. PMID: 31407850 Free PMC article.
-
Sulfated Non-Saccharide Glycosaminoglycan Mimetics as Novel Drug Discovery Platform for Various Pathologies.Curr Med Chem. 2020;27(21):3412-3447. doi: 10.2174/0929867325666181120101147. Curr Med Chem. 2020. PMID: 30457046 Free PMC article. Review.
-
The Plasma Factor XIII Heterotetrameric Complex Structure: Unexpected Unequal Pairing within a Symmetric Complex.Biomolecules. 2019 Nov 21;9(12):765. doi: 10.3390/biom9120765. Biomolecules. 2019. PMID: 31766577 Free PMC article.
-
Factor XIIIa inhibitors as potential novel drugs for venous thromboembolism.Eur J Med Chem. 2020 Aug 15;200:112442. doi: 10.1016/j.ejmech.2020.112442. Epub 2020 May 18. Eur J Med Chem. 2020. PMID: 32502864 Free PMC article. Review.
-
Ethacrynic acid is an inhibitor of human factor XIIIa.BMC Pharmacol Toxicol. 2022 Jun 1;23(1):35. doi: 10.1186/s40360-022-00575-5. BMC Pharmacol Toxicol. 2022. PMID: 35642005 Free PMC article.
References
-
- Henry BL, Desai UR . Anticoagulants In Burger’s Medicinal Chemistry, John Wiley and Sons: New York, 2010; 365–408.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

