Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation
- PMID: 27467537
- PMCID: PMC7228592
- DOI: 10.1097/TP.0000000000001343
Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation
Abstract
Background: Donation after circulatory death (DCD) is current clinical practice to increase the donor pool. Deleterious effects on renal graft function are described for hypothermic preservation. Therefore, current research focuses on investigating alternative preservation techniques, such as normothermic perfusion.
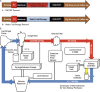
Methods: We compared continuous pressure-controlled normothermic ex vivo kidney perfusion (NEVKP) with static cold storage (SCS) in a porcine model of DCD autotransplantation. After 30 minutes of warm ischemia, right kidneys were removed from 30-kg Yorkshire pigs and preserved with 8-hour NEVKP or in 4°C histidine-tryptophan-ketoglutarate solution (SCS), followed by kidney autotransplantation.
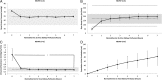
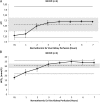
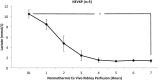
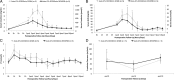
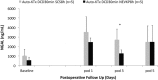
Results: Throughout NEVKP, electrolytes and pH values were maintained. Intrarenal resistance decreased over the course of perfusion (0 hour, 1.6 ± 0.51 mm per minute vs 7 hours, 0.34 ± 0.05 mm Hg/mL per minute, P = 0.005). Perfusate lactate concentration also decreased (0 hour, 10.5 ± 0.8 vs 7 hours, 1.4 ± 0.3 mmol/L, P < 0.001). Cellular injury markers lactate dehydrogenase and aspartate aminotransferase were persistently low (lactate dehydrogenase < 100 U/L, below analyzer range; aspartate aminotransferase 0 hour, 15.6 ± 9.3 U/L vs 7 hours, 24.8 ± 14.6 U/L, P = 0.298). After autotransplantation, renal grafts preserved with NEVKP demonstrated lower serum creatinine on days 1 to 7 (P < 0.05) and lower peak values (NEVKP, 5.5 ± 1.7 mg/dL vs SCS, 11.1 ± 2.1 mg/dL, P = 0.002). The creatinine clearance on day 4 was increased in NEVKP-preserved kidneys (NEVKP, 39 ± 6.4 vs SCS, 18 ± 10.6 mL/min; P = 0.012). Serum neutrophil gelatinase-associated lipocalin at day 3 was lower in the NEVKP group (1267 ± 372 vs 2697 ± 1145 ng/mL, P = 0.029).
Conclusions: Continuous pressure-controlled NEVKP improves renal function in DCD kidney transplantation. Normothermic ex vivo kidney perfusion might help to decrease posttransplant delayed graft function rates and to increase the donor pool.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures
Comment in
-
Transplantation: Continuous normothermic ex vivo perfusion improves kidney graft function.Nat Rev Nephrol. 2016 Oct;12(10):580. doi: 10.1038/nrneph.2016.128. Epub 2016 Aug 16. Nat Rev Nephrol. 2016. PMID: 27526866 No abstract available.
References
-
- Wolfe RA, Ashby VB, Milford EL. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant N Engl J Med 1999. 3411725–1730 - PubMed
-
- Tonelli M, Wiebe N, Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes Am J Transplant 2011. 112093–2109 - PubMed
-
- Ingsathit A, Kamanamool N, Thakkinstian A. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis Transplantation 2013. 95943–948 - PubMed
-
- Meier-Kriesche HU, Port FK, Ojo AO. Effect of waiting time on renal transplant outcome Kidney Int 2000. 581311–1317 - PubMed
-
- Rana A, Gruessner A, Agopian VG. Survival benefit of solid-organ transplant in the United States JAMA Surg 2015. 150252–259 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

