Modulation of Phosphopeptide Fragmentation via Dual Spray Ion/Ion Reactions Using a Sulfonate-Incorporating Reagent
- PMID: 27467576
- PMCID: PMC5477467
- DOI: 10.1021/acs.analchem.6b01901
Modulation of Phosphopeptide Fragmentation via Dual Spray Ion/Ion Reactions Using a Sulfonate-Incorporating Reagent
Abstract
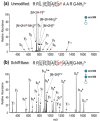
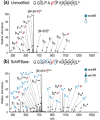
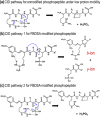
The labile nature of phosphoryl groups has presented a long-standing challenge for the characterization of protein phosphorylation via conventional mass spectrometry-based bottom-up proteomics methods. Collision-induced dissociation (CID) causes preferential cleavage of the phospho-ester bond of peptides, particularly under conditions of low proton mobility, and results in the suppression of sequence-informative fragmentation that often prohibits phosphosite determination. In the present study, the fragmentation patterns of phosphopeptides are improved through ion/ion-mediated peptide derivatization with 4-formyl-1,3-benezenedisulfonic acid (FBDSA) anions using a dual spray reactor. This approach exploits the strong electrostatic interactions between the sulfonate moieties of FBDSA and basic sites to facilitate gas-phase bioconjugation and to reduce charge sequestration and increase the yield of phosphate-retaining sequence ions upon CID. Moreover, comparative CID fragmentation analysis between unmodified phosphopeptides and those modified online with FBDSA or in solution via carbamylation and 4-sulfophenyl isothiocyanate (SPITC) provided evidence for sulfonate interference with charge-directed mechanisms that result in preferential phosphate elimination. Our results indicate the prominence of charge-directed neighboring group participation reactions involved in phosphate neutral loss, and the implementation of ion/ion reactions in a dual spray reactor setup provides a means to disrupt the interactions by competing hydrogen-bonding interactions between sulfonate groups and the side chains of basic residues.
Figures


References
-
- Cohen P. Nat Cell Biol. 2002;4:E127–E130. - PubMed
-
- Ubersax JA, Ferrell JE., Jr Nat Rev Mol Cell Biol. 2007;8:530–541. - PubMed
-
- Johnson LN. Biochem Soc Trans. 2009;37:627–641. - PubMed
-
- Hunter T. Cell. 1995;80:225–236. - PubMed
-
- Lahiry P, Torkamani A, Schork NJ, Hegele RA. Nat Rev Genet. 2010;11:60–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

