MRI as a Novel In Vivo Approach for Assessing Structural Changes of Chlamydia Pathology in a Mouse Model
- PMID: 27467585
- PMCID: PMC4965011
- DOI: 10.1371/journal.pone.0160055
MRI as a Novel In Vivo Approach for Assessing Structural Changes of Chlamydia Pathology in a Mouse Model
Abstract
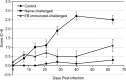
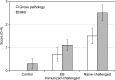
Chlamydia trachomatis is among the most prevalent of sexually transmitted diseases. While Chlamydia infection is a reportable event and screening has increased over time, enhanced surveillance has not resulted in a reduction in the rate of infections, and Chlamydia infections frequently recur. The development of a preventative vaccine for Chlamydia may be the only effective approach for reducing infection and the frequency of pathological outcomes. Current vaccine research efforts involve time consuming and/or invasive approaches for assessment of disease state, and MRI presents a clinically translatable method for assessing infection and related pathology both quickly and non-invasively. Longitudinal T2-weighted MRI was performed over 63 days on both control or Chlamydia muridarum challenged mice, either with or without elementary body (EB) immunization, and gross necropsy was performed on day 65. A scoring system was developed to assess the number of regions affected by Chlamydia pathology and was used to document pathology over time and at necropsy. The scoring system documented increasing incidence of pathology in the unimmunized and challenged mice (significantly greater compared to the control and EB immunized-challenged groups) by 21 days post-challenge. No differences between the unchallenged and EB immunized-challenged mice were observed. MRI scores at Day 63 were consistently higher than gross necropsy scores at Day 65, although two of the three groups of mice showed no significant differences between the two techniques. In this work we describe the application of MRI in mice for the potential evaluation of disease pathology and sequelae caused by C. muridarum infection and this technique's potential for evaluation of vaccines for Chlamydia.
Conflict of interest statement
Figures
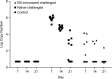
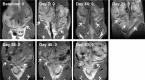
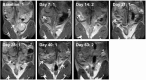
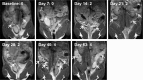
References
-
- Centers for Disease Control and Prevention. 2011 Sexually Transmitted Disease Surveillance: Chlamydia. Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention, Centers for Disease Control and Prevention. 2012;12:13 Available: http://www.cdc.gov/std/stats11/chlamydia.htm.
-
- Centers for Disease Control and Prevention. CDC Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep. 2011;60(12):370–3. - PubMed
-
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. February 2005; 5(2): 149–61. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

