Characterization of Venom and Oviduct Components of Parasitoid Wasp Asobara japonica
- PMID: 27467595
- PMCID: PMC4965004
- DOI: 10.1371/journal.pone.0160210
Characterization of Venom and Oviduct Components of Parasitoid Wasp Asobara japonica
Abstract
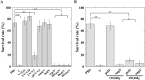
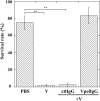
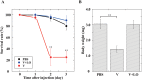
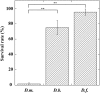
During natural parasitization, Asobara japonica wasps introduce lateral oviduct (LO) components into their Drosophila hosts soon after the venom injection to neutralize its strong toxicity; otherwise, the host will die. Although the orchestrated relationship between the venom and LO components necessary for successful parasitism has attracted the attention of many researchers in this field, the molecular natures of both factors remain ambiguous. We here showed that precipitation of the venom components by ultracentrifugation yielded a toxic fraction that was inactivated by ultraviolet light irradiation, boiling, and sonication, suggesting that it is a virus-like entity. Morphological observation of the precipitate after ultracentrifugation showed small spherical heterogeneous virus-like particles 20-40 nm in diameter. The venom's detrimental effect on D. melanogaster larvae was not directly neutralized by the LO components but blocked by a hemolymphal neutralizing factor activated by the LO factor. Furthermore, we found that A. japonica venom and LO components acted similarly on the larvae of the common cutworm Spodoptera litura: the venom injection caused mortality but coinjection of the LO factor protected S. litura larvae from the venom's toxicity. In contrast, D. ficusphila and D. bipectinata, which are closely related to D. melanogaster but non-habitual host species of A. japonica, were not negatively affected by A. japonica venom due to an intrinsic neutralizing activity in their hemolymph, indicating that these species must have acquired a neutralizer of A. japonica venom during evolution. These results give new insights into the characteristics of both the venom and LO components: A. japonica females have utilized the virus-like toxic venom factor to exploit a wider range of host species after the evolutionary process enabled them to use the LO factor for activation of the host hemolymph neutralizer precursor, although the non-habitual host Drosophila species possess an active intrinsic neutralizer in their hemolymph.
Conflict of interest statement
Figures


Similar articles
-
Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism.Sci Adv. 2025 Jan 31;11(5):eadq8771. doi: 10.1126/sciadv.adq8771. Epub 2025 Jan 29. Sci Adv. 2025. PMID: 39879297 Free PMC article.
-
Venom components of Asobara japonica impair cellular immune responses of host Drosophila melanogaster.Arch Insect Biochem Physiol. 2013 Jun;83(2):86-100. doi: 10.1002/arch.21093. Epub 2013 Apr 19. Arch Insect Biochem Physiol. 2013. PMID: 23606512
-
Deadly venom of Asobara japonica parasitoid needs ovarian antidote to regulate host physiology.J Insect Physiol. 2010 Jan;56(1):35-41. doi: 10.1016/j.jinsphys.2009.09.001. J Insect Physiol. 2010. PMID: 19769980
-
Components of Asobara venoms and their effects on hosts.Adv Parasitol. 2009;70:217-32. doi: 10.1016/S0065-308X(09)70008-9. Adv Parasitol. 2009. PMID: 19773072 Review.
-
Variation of Leptopilina boulardi success in Drosophila hosts: what is inside the black box?Adv Parasitol. 2009;70:147-88. doi: 10.1016/S0065-308X(09)70006-5. Adv Parasitol. 2009. PMID: 19773070 Review.
Cited by
-
Mapping the global distribution of invasive pest Drosophila suzukii and parasitoid Leptopilina japonica: implications for biological control.PeerJ. 2023 Apr 24;11:e15222. doi: 10.7717/peerj.15222. eCollection 2023. PeerJ. 2023. PMID: 37123003 Free PMC article.
-
Multi-omic approach to characterize the venom of the parasitic wasp Cotesia congregata (Hymenoptera: Braconidae).BMC Genomics. 2025 Apr 30;26(1):431. doi: 10.1186/s12864-025-11604-y. BMC Genomics. 2025. PMID: 40307720 Free PMC article.
-
Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism.Sci Adv. 2025 Jan 31;11(5):eadq8771. doi: 10.1126/sciadv.adq8771. Epub 2025 Jan 29. Sci Adv. 2025. PMID: 39879297 Free PMC article.
-
A Horizontally Transferred Autonomous Helitron Became a Full Polydnavirus Segment in Cotesia vestalis.G3 (Bethesda). 2017 Dec 4;7(12):3925-3935. doi: 10.1534/g3.117.300280. G3 (Bethesda). 2017. PMID: 29042411 Free PMC article.
-
Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps.Biology (Basel). 2021 Jan 12;10(1):50. doi: 10.3390/biology10010050. Biology (Basel). 2021. PMID: 33445639 Free PMC article. Review.
References
-
- Vinson SB, Iwantsch GF. Host regulation by insect parasitoids. Quarterly Rev Biol 1980;55: 145–159..
-
- Vinson SB, Pennacchio F, Lanzrein B. Interactions between parasitoids and their hosts: an introduction and perspectives. J Insect Physiol 1998;44: 701–702.
-
- Hayakawa Y. Cellular immunosuppressive protein in the plasma of parasitized insect larvae. J Biol Chem 1994;269: 14536–14540. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

