Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients
- PMID: 27467945
- PMCID: PMC4910752
- DOI: 10.1080/2162402X.2015.1129483
Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients
Abstract
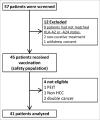
The recurrence rates of Hepatocellular carcinoma (HCC) are high, necessitating novel and effective adjuvant therapies. Therefore, we conducted a phase II study of glypican-3 (GPC3) peptide vaccine as an adjuvant therapy for HCC patients. Forty-one patients with initial HCC who had undergone surgery or radiofrequency ablation (RFA) were analyzed in this phase II, open-label, single-arm trial. Ten vaccinations were performed for 1 y after curative treatment. We also investigated case-control subjects, where selected patients treated surgically during the same period were analyzed. The expression of GPC3 in the available primary tumors was determined by immunohistochemical analysis. Six patients received RFA therapy while 35 received surgery. The recurrence rate tended to be lower in the 35 patients treated with surgery plus vaccination compared to 33 patients who underwent surgery alone (28.6% vs. 54.3% and 39.4% vs. 54.5% at 1 and 2 y, respectively; p = 0.346, 0.983). Twenty-five patients treated with surgery and vaccination had GPC3-positive tumors; the recurrence rate in this group was significantly lower compared to that in 21 GPC3-positive patients who received surgery only (24% vs. 48% and 52.4% vs. 61.9% at 1 and 2 y, respectively; p = 0.047, 0.387). The GPC3 peptide vaccine improved the 1-y recurrence rate in patients with GPC3-positive tumors. This study demonstrated that GPC3 expression by the primary tumor may be used as a biomarker in a putative larger randomized clinical trial to determine the efficacy of the GPC3-derived peptide vaccine.
Keywords: Adjuvant therapy; CTL; HCC; glypican-3; peptide vaccine.
Figures



References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107 - DOI - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557-76; PMID:17570226; http://dx.doi.org/10.1053/j.gastro.2007.04.061 - DOI - PubMed
-
- Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg 2000; 191:381-8; PMID:11030243; http://dx.doi.org/10.1016/S1072-7515(00)00700-6 - DOI - PubMed
-
- Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, et al. Radiofrequency Ablation for Hepatocellular Carcinoma: 10-Year Outcome and Prognostic Factors. Am J Gastroenterol 2012; 107:569-77; PMID:22158026; http://dx.doi.org/10.1038/ajg.2011.425 - DOI - PMC - PubMed
-
- Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology 1997; 25:87-92; PMID:8985270; http://dx.doi.org/10.1002/hep.510250116 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
