Serum anti-MDM2 and anti-c-Myc autoantibodies as biomarkers in the early detection of lung cancer
- PMID: 27467958
- PMCID: PMC4910725
- DOI: 10.1080/2162402X.2016.1138200
Serum anti-MDM2 and anti-c-Myc autoantibodies as biomarkers in the early detection of lung cancer
Abstract
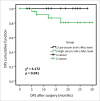
This study aims to investigate the clinical significance of serum autoantibodies against MDM2 and c-Myc and evaluate their feasibility in the immunodiagnosis of lung cancer. 50 sera samples with 43 available paired lung cancer tissue and adjacent normal tissue slides with follow up information and 44 sera from normal human controls (NHC) were used in the research group. Another 62 lung cancer sera and 43 NHC sera were used in the validation group. The results of IHC showed that MDM2 and c-Myc protein were overexpressed in lung cancer tissues compared to adjacent normal tissues (p < 0.001). Likewise, significantly higher levels of serum autoantibodies against MDM2 and c-Myc were found in lung cancer compared to NHC both in research and validation groups. Further analysis on IHC and ELISA results showed that serum level of autoantibodies against these two TAAs were positively associated with tissue staining scores (both p < 0.05). The area under curve (AUC) values of anti-MDM2 and anti-cMyc autoantibodies for discriminating lung cancers from NHC were 0.698 and 0.636 in research group, 0.777 and 0.815 in the validation group, respectively. Both anti-MDM2 and anti-c-Myc autoantibodies can discriminate stage I lung cancer patients from NHC with AUC values of 0.703 and 0.662. Kaplan-Meier analysis showed that higher level of serum anti-c-Myc autoantibodies was significantly related to shortened disease-free survival (DFS) (p = 0.041). In conclusion, our finding suggested that serum MDM2 and c-Myc autoantibodies may have the potential to serve as non-invasive diagnostic biomarkers in patients with lung cancer.
Keywords: Autoantibody; MDM2; biomarker; c-Myc; immunodiagnosis; lung cancer; tumor-associated antigen.
Figures





References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29; PMID:25559415; http://dx.doi.org/10.3322/caac.21254 - DOI - PubMed
-
- Midthun DE. Early diagnosis of lung cancer. F1000 Prime Rep 2013; 5:12; PMID:23585930; http://dx.doi.org/10.12703/P5-12 - DOI - PMC - PubMed
-
- Arkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, Forman D. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010; 127:2918–27; PMID:21351270; http://dx.doi.org/10.1002/ijc.25517 - DOI - PubMed
-
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging C et al.. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007; 2:706–14; PMID:17762336; http://dx.doi.org/10.1097/JTO.0b013e31812f3c1a - DOI - PubMed
-
- Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z et al.. SEER cancer statistics review, 1975–2012. National Cancer Institute; Bethesda, MD: http://seercancergov/csr/1975_2012/2015
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
